|
Abstract
Background: In the present study we developed, evaluated in
volunteers, and clinically validated an image
acquisition stabilizer (IAS) for Sidestream Dark Field (SDF)
imaging(Sidestream Dark Field(SDF),SDF
imaging device). Methods: The IAS is a stainless steel sterilizable ring
which fits around the SDF probe tip. The IAS creates adhesion to
the imaged tissue by application of negative pressure. The
effects of the IAS on the sublingual microcirculatory flow
velocities, the force required to induce pressure artifacts
(PA), the time to acquire a stable image, and the duration of
stable imaging were assessed in healthy volunteers. To
demonstrate the clinical applicability of the SDF setup in
combination with the IAS, simultaneous bilateral sublingual
imaging of the microcirculation were performed during a lung
recruitment maneuver (LRM) in mechanically ventilated critically
ill patients. One SDF imaging device(Sidestream
Dark Field(SDF),Sidestream Dark Field (SDF)
imaging) was operated handheld; the second
was fitted with the IAS and held in position by a mechanic arm.
Lateral drift, number of losses of image stability and duration
of stable imaging of the two methods were compared.
Results: Five healthy volunteers were studied. The IAS did not
affect microcirculatory flow velocities. A significantly greater
force had to applied onto the tissue to induced PA with compared
to without IAS (0.25 �� 0.15 N without vs. 0.62 �� 0.05 N with the
IAS, p < 0.001). The IAS ensured an increased duration of a
stable image sequence (8 �� 2 s without vs. 42 �� 8 s with the IAS,
p < 0.001). The time required to obtain a stable image sequence
was similar with and without the IAS. In eight mechanically
ventilated patients undergoing a LRM the use of the IAS resulted
in a significantly reduced image drifting and enabled the
acquisition of significantly longer stable image sequences (24 ��
5 s without vs. 67 �� 14 s with the IAS, p = 0.006).
Conclusions: The present study has validated the use of an IAS
for improvement of SDF imaging by demonstrating that the IAS did
not affect microcirculatory perfusion in the microscopic field
of view. The IAS improved both axial and lateral SDF image(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device)
stability and thereby increased the critical force required to
induce pressure artifacts. The IAS ensured a significantly
increased duration of maintaining a stable image sequence.
Background
Orthogonal Polarization Spectral (OPS) imaging and its successor
Sidestream Dark Field (SDF) imaging are opti¬cal techniques
allowing microscopic assessment of microcirculatory density and
perfusioninclinicalset¬tings [1,2]. These non-invasive
intravital imaging modal¬ities have been used in studies for
monitoring the severity of shock and efficacy of resuscitation
in various patient groups [3-6]. However, as both OPS and SDF
imaging(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) technologies are incorporated into hand-held microscopes
some operational issues arise in terms of axial and lateral
instability of the microscope probes, potentially causing
pressure artifacts and image drifting, respectively.
Reductions in sublingual microcirculatory density and perfusion
have been associated with patient morbidity and mortality [6].
Correcting these microcirculatory parameters has become the
focus of new clinical studies aiming at resuscitating the
microcirculation rather than the macrocirculation, using
vasoactive agents such as nitroglycerin [7,8]. Hence,
microcirculatory images are gaining a more prominent role in
clinical monitoring and their accurate interpretation is
essential and relies .
heavily on the quality of the images [9,10]. In this light, the
current microcirculatory image acquisition guide¬lines dictate a
minimal recording time of 20 s to allow adequate analysis of
microcirculatory density and perfu¬sion [11]. Image drifting,
due to the difficulty in holding the tip of the device in one
place however, makes this particularly difficult both in sedated
and in awake patients. Furthermore, pressure artifacts caused by
the physical contact and pressure of the microscope probe to the
mucosal tissue can alter mucosal capillary blood flow thereby
limit the use of the captured images for determination of
microcirculatory perfusion.
Sublingual microcirculatory density and perfusion were monitored
using an SDF imaging device��Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF)�� (Microvision Medical BV, Amsterdam,
the Netherlands). A detailed description of the SDF technology
is provided elsewhere [13]. Briefly, in SDF imaging, the tissue
is illuminated with green light emitting diodes (LEDs)
concentrically placed around the central microscopy objective to
pro¬vide SDF illumination. The lens system in the core of the
objective is optically isolated from the illuminating outer ring
thus preventing the microcirculatory image from contamination by
tissue surface reflections. To further improve the imaging of
flowing erythrocytes, the SDF device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) provides pulsed
illumination in synchrony with the camera frame rate. This
stroboscopic imaging, (partially) prevents smearing of flowing
erythrocytes and motion-induced blurring of capillaries due to
the short illumination intervals [13].
Lindert et al. addressed these technical issues asso¬ciated with
hand-held microscopy before by developing an image acquisition
stabilizer (IAS) for the OPS imaging device [12]. Their IAS
consisted of a ring placed around the tip of the OPS probe
through which negative pressure was applied securing the IAS
onto the mucosal tissue. The negative pressure appeared not to
influence flow patterns of the microcirculation within the
micro¬scopic field. However, whether the IAS minimized image
drift or induction of pressure artifacts was not evaluated. In
addition their IAS was not validated in terms of clinical
applicability and utility, including the ease with which the
device could be sterilized and cleaned for multiple uses as well
as fitting piping of vacuum sources available at the bed-side.
In the present study we developed, evaluated, and vali¬dated an
IAS for the SDF device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device). In a study by Goedhart et al., the SDF
imaging device was shown to provide microcirculatory images of
superior quality with respect to the OPS device [13]. In
combination with an IAS, this microcirculatory imaging setup
should provide high quality microcirculatory images of
sufficient duration and stability. The IAS was designed and
fabricated to adhere to clinical requirements. The application
of the IAS was validated by measuring 1) the effects of
applica¬tion of peripheral negative pressure on microcirculatory
perfusion, 2) the force required for induction of pressure
artifacts with and without the IAS, 3) the time required to
attain astableimage,and 4) thetimethatastable image could be
maintained. Then, to demonstrate the clinical applicability of
the SDF setup with the IAS, simultaneous bilateral sublingual
SDF measurements were conducted in critically ill patients
undergoing a standard lung recruitment maneuver with one
hand¬held SDF imaging device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF)) and one
SDF imaging device mountedin a mechanical arm
and equipped with the IAS whereby stability of acquired images
was evaluated.
Methods
The study protocol was approved by the local medical ethics
committee of the Medical Center of Leeuwarden. Written informed
consent was obtained from all studied subjects respectively
their closest relatives. The study wasdoneincompliancewith the
principles established in the Helsinki Declaration.
Sidestream dark field image acquisition and analysis The
obtained microcirculatory images (one per time point) were
stored on DVI tape and saved onto a com¬puter in DV-AVI file
format. The microvessels in the SDF images(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) were analyzed blinded
for microvascular diameters and blood flow velocity using a
computer software package, MAS (Microvascular Analysis Soft¬ware,
Microvision Medical BV) [14]. Furthermore, image drifting was
measured as the translation (in pixels) of an image with respect
to the first image of a video sequence as depicted in fig. 1.
Image drift of 40 pixels, in either x-or y-direction, was
arbitrarily chosen as a cut off for a stable video sequence.
Image Acquisition Stabilizer (IAS)
The basis of the IAS is a hollow stainless steel cylinder which
fits snugly around the tip of the disposable sterile cap of the
SDF probe (Fig. 2). The outer cylinder of the IAS can be
unscrewed from the inner cylinder to allow cleaning and
sterilization of the IAS. Furthermore, the
IASisdesignedsuchthatitleavesa100 ��m space between the tip of
the SDF probe(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) cover and the tissue and thereby relieves the
imaged tissue area from pres¬sure without losing image focus.
Negative pressure can be applied by use of a readily available
bed side vacuum source and a pressure regulator (Digital Vacuum
Regu¬lator, Amvex, Richmond Hill, Canada) and applied to
thetissuevia 20 concavechannels.Toprevent fluid from reaching
the vacuum regulator, a fluid trap (Argyle Lukens Specimen
Container; Kendall/Tyco Healthcare/ Covidien; Wollerau;
Switzerland) is interposed between the IAS and the vacuum
regulator. In accordance to previous published values [12], we
found the best fit for
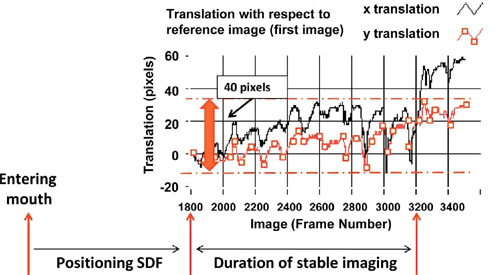
Figure 1 Assessment of image stability. Image drifting was
measured as the translation (in pixels) of an image with respect
to the first image of a video sequence. Image drift of 40
pixels, in either x-or y-direction, was arbitrarily chosen as a
cut off for a stable video sequence.
Validation protocol
For the validation part of the study, five experienced SDF
imaging(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) operators (i.e., >1 year of SDF imaging training in
supervision of the Department of Transla¬tional Physiology,
Academic Medical Center, Amster¬dam) measured sublingual
microvascular perfusion in five healthy awake volunteers (n =
5). In case of secre¬tions, the recording was stopped and the
awake subjects were asked to swallow. Then, the probe was
reposi¬tioned and recording was started again. No wipes or
cotton sticks were used to absorb saliva as this can cause
microscopic bleeding and significantly alter the measurements.
First, the effects of applying peripheral negative pres¬sure on
the microcirculatory blood flow velocities [��m/s] were evaluated
by switching the negative pressure source on and off during a
single video sequence while the SDF device was hand-held.
Second, the SDF imaging device was mounted in a force-measuring
mechanical arm (Pesola AG, Baar, Switzerland) and the force [N]
required to induce pressure artifacts (i.e., stopped or slowed
venular flow) was determined with and without the IAS by
systematically increasing the force applied by the SDF probe(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device)
onto the sublingual tissue. Third, the time [s] required for
obtaining a stable image sequence and, fourth, the duration [s]
of maintaining that stable image sequence were measured.
Clinical protocol
To demonstrate the clinical applicability of the SDF setup with
the IAS, simultaneous bilateral sublingual SDF measurements were
conducted in eight intensive care patients undergoing a standard
lung recruitment maneuver with one hand-held SDF device and one
SDF device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) mounted in a mechanical arm and equipped with the IAS.
This procedure, with a stepwise increment of tidal volume, was
chosen in order to create extensive movement artifacts in the
sublingual imaged areas. When both SDF devices were acquiring
stable images, without pressure artifacts, continuous recording
of the video image was started. The lung recruitment maneu¬ver
was performed by increasing the inspiratory pressure level to a
target of 40 cmH2O, followed by gradually reducing the pressure
until the baseline ventilator set¬tings were regained. The
fraction of inspired oxygen and positive end-expiratory pressure
were maintained at 40% and 12 cmH2O, respectively, throughout
the procedure.
SDF images(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) were recorded non-stop from 1 min before till 1 min
after the recruitment maneuver and the recorded SDF video
sequences were randomized and
analyzed off-line for lateral image drift [��m], drifting
velocity [��m/s], and number of loss of image stability (i.e.,
image drift of > 40 pixels, Fig. 1), the duration [s] that a
stable image could be maintained.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version
5.0 for Windows (GraphPad Software, San Diego, CA, USA). To test
data sets for (non-)parametric distributions a D��Agostino-Pearson
omnibus normality test was applied. Comparative analysis between
data sets was performed with the unpaired Student��st-testor the
Mann-Whitney U test and comparative analysis between time points
was performed with the paired Student��s t-test or the Wilcoxon
signed rank test, as appropriate. Differences with a p-value of
< 0.05 were considered statistically significant. Results are
reported as mean �� SEM.
Results
Validation protocol
The application of peripheral negative pressure thought the IAS
did not affect the blood flow velocity in small (< 20 ��m),
medium (20-50 ��m), and large (> 50 ��m) microvessels (Fig. 3).
The force applied by the SDF imaging(SDF imaging device) probe onto the mucosal
tissue required to induce pressure artifacts (i.e., stopped or
slowed venular flow) was significantly (p < 0.001) higher with
the IAS (0.62 ��
0.05 N) compared to without the IAS (0.25 �� 0.15 N).
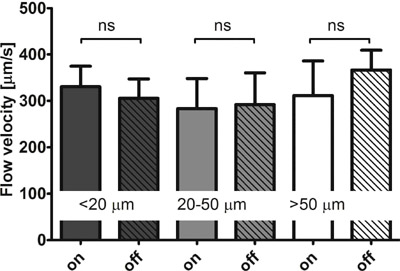
Figure 3 Influence on microcirculatory flow velocities. The flow
velocity [��m/s] in small (< 20 ��m), medium (20-50 ��m), and large
(> 50 ��m) microvessels measured in Sidestream Dark Field (SDF)
(Sidestream Dark Field (SDF)
imaging,SDF imaging device)video sequences of 5 healthy volunteers while the negative
pressure source of the image acquisition stabilizer (IAS) was
switched on and off. In all microvessels p = ns for negative
pressure source on versus off.
The time required to obtain a stable SDF image (SDF imaging device)sequence was
similar (p = 0.12) with (99 �� 20 s) and without the IAS (150 ��
25 s). The duration of maintain¬ingthatstableimage
wasapproximatelyfivetimes longer with the IAS: 8 �� 2 s without
the IAS and 42 �� 8 s with the IAS (p < 0.001).
Clinical protocol
In the eight patients undergoing a lung recruitment maneuver
(four male and four female), aged 66 �� 5 years, the APACHE II,
APACHE IV, SOFA scores were 19 �� 2, 75 �� 10, and 8 �� 1 points
respectively. Four patients were diagnosed with abdominal
sepsis, one with coma after cardiac arrest, two had undergone
cardiovas¬cular surgery, and one head and neck surgery.
During the lung recruitment maneuver, inspiratory pressure level
was increased from 11.4 �� 0.8 cmH2Oto 43 �� 2 cmH2O (p < 0.001).
Tidal volume rose accord¬ingly from 423 �� 23 ml (baseline) to
1208 �� 90 ml (p < 0.001) during the lung recruitment maneuver
and returned to 477 �� 30 ml after the maneuver (p = 0.017 vs.
baseline).
Continuous recording of the SDF video image was started prior to
the lung recruitment maneuver when both SDF devices (Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device)were
acquiring stable images, without pressure artifacts. During the
procedure, image drift in x¬and y-direction was 8.9 �� 2.6 and
10.1 �� 2.0 mm respec¬tively without the IAS, while the drift was
reduced to 3.4 �� 0.9 (p = 0.066) and 3.8 �� 1.3 mm (p = 0.018)
with the IAS. Drift velocity in x-and y-direction was 15.5 �� 3.9
and 18.4 �� 3.3 ��m/s respectively without the IAS, which was
reduced to 5.4 �� 1.5 (p = 0.032) and 5.6 �� 1.7 ��m/s (p = 0.004)
with the IAS. Image drift of > 20 pixels within one video
sequence occurred 50 �� 13 times with¬out the IAS and 8 �� 3 times
(p < 0.001) with the IAS. The maximum duration of stable imaging
during the lung recruitment maneuver was 24 �� 5 s without the
IAS and 66 �� 14 s (p = 0.006) with the IAS.
Discussion
In the present study we developed, evaluated, and vali¬dated an
IAS for the SDF device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device). The IAS was based on creating adherence
of the SDF probe to the sublingual tissue by applying negative
pressure to the periphery of the microscopic field of view. The
main findings were that: 1) the IAS did not affect
microcirculatory perfusion in the SDF imaging field of view; 2)
the IAS prevented pressure artifacts up to a significantly
greater force applied by the SDF(SDF imaging device) probe onto the tissue; 3) the
time required to obtain a stable image sequence was similar with
and without the IAS; and 4) the duration of main¬taining that
stable image sequence was significantly increased with the IAS.
Ultimately, to demonstrate the clinical applicability of the SDF
setup with the IAS,
simultaneous bilateral sublingual SDF measurements were
conducted in intensive care patients undergoing a standard lung
recruitment maneuver with one handheld SDF device(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) and one SDF
device mounted in a mechani¬cal arm and equipped with the IAS.
It was shown that the IAS significantly reduced image drifting
and enabled the acquisition of significantly longer image
sequences. A final and important finding is also that we showed,
in proof of concept, that with the IAS it is possible to
per¬form a measurement without the need for an operator by
mounting the device on a mechanical arm, leaving the operator
free to perform a clinical maneuver.
The design of the IAS presented here is based on an IAS
developed by Lindert et al. for OPS imaging, includ¬ing the
negative pressure level of ��100 mmHg [12]. To show that
application of peripheral negative pressure did not affect
microcirculatory perfusion in the SDF imaging (Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) field of view Lindert et al. measured blood flow velocities in venules and
arterioles. They found that the velocities did not change after
switching the negative pressure source on. In the present study,
for validation purposes, we investigated the effects on blood
flow velo¬cities in small, medium, and large microvessels in
five healthy volunteers and provided evidence that indeed
microcirculatory perfusion is not affected by application of
negative pressure though the IAS. These experiments demonstrated
that the IAS is a valid method for SDF image stabilization, not
affecting microcirculatory perfusion in the microscopic field of
view.
It has been well established that pressure artifacts are easily
induced and diminish the reliability of SDF mea¬surements of
microcirculatory perfusion [6,11]. This appreciation known from
daily application of SDF ima¬ging is confirmed and highlighted
by the low force level required to induce pressure artifacts
found in the pre¬sent study. The SDF imaging device has a mass
of approximately 360 g. The critical force onto the sublin¬gual
tissue without the IAS, at which pressure artifacts are induced,
was found to amount approximately 1/6 of the mass of the SDF
device. Hence, physical feedback is impossible for SDF(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) operators
and visual feedback in the microcirculatory images is necessary
to avoid excessive pressure. In fact, most SDF operators use
visual feed¬back to gauge the pressure exerted by the SDF probe
on the imaged microcirculation as exemplified in a recent
publication [6]. De Backer et al., defined the critical pressure
inducing perfusion artifacts at the point where venular flow
either stopped or significantly slowed down [11]. Using a
similar cut-off in the present study we were able to show that
the larger surface contact area created by the presence of the
IAS resulted in an approximately five times greater force
required for the induction of pressure artifacts. This
significantly improved SDF image acquisition.
Another important advantage of using an IAS for SDF imaging is
that it allows acquisition of longer and more stable SDF(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) image
sequences. Previous studies reported that SDF measurements have
low intra-and inter-observer variability [3] and that
microcirculatory density and perfu¬sion vary highly per site and
in time [15]. Hence, studying the microcirculation under
pathophysiological conditions requires multiple measurements per
time point in order to eliminate this site-and time-dependency
of the obtained results. The current microcirculatory image
acquisition guidelines dictate that microcirculatory density and
perfu¬sion should be measured in 3-5 sites per time point to
allow adequate interpretation of the results [11]. Further¬more,
according to these guidelines, the length of each SDF image(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device)
sequence should be > 20 s. This was proven to be rather
difficult without the IAS and fairly easy with the IAS. An
alternative for multiple measurements to determine the
microcirculatory state at a certain time point, continuous
measurements of microcirculatory perfusion and density during a
clinical maneuver or intervention (e.g., nitroglycerin
administration) would allow direct assessment of their effects
on the micro¬circulation. The presented IAS would potentially
enable such studies.
Non-invasive intravital imaging modalities, such as OPS and SDF
imaging(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device), have been used in studies for monitoring the severity
of shock and efficacy of resusci¬tation and alterations in
sublingual microcirculatory density and perfusion have been
associated with patient morbidity and mortality [3,6,16].
��Normalizing�� microcir¬culatory density and perfusion has become
focus of new clinical studies and microcirculatory images are
gaining a more prominent role in clinical monitoring. Adequate
interpretation of microcirculatory images is essential and
relies heavily on the quality of the images, in terms of axial
and lateral stability. In the present study we showed that the
IAS improves both axial and lateral sta¬bility of the acquired
microcirculatory images and signif¬icantly reduced pressure
artifacts and image drifting.
Conclusions
Thepresent studyhas validated theuse of an IASfor improvement of
SDF imaging by demonstrating that the application of peripheral
negative pressure though the IAS does not affect
microcirculatory perfusion in the microscopic field of view.
Furthermore, the IAS was shown to improve both axial and lateral
SDF image(Sidestream Dark Field (SDF)
imaging,Sidestream Dark Field(SDF),SDF imaging device) sta¬bility and thereby increased the critical force
required to induce microcirculatory pressure artifacts and
increased the duration of stable image acquisition.
Key Messages
• The application of peripheral negative pressure though the
image acquisition stabilizer (IAS) for
improvement of SDF imaging did not affect micro¬circulatory
perfusion in the microscopic field of view.
•The IAS improved both axial and lateral SDF image stability and
thereby increased the critical force required to induce
microcirculatory pressure artifacts.
•The IAS increased the duration of stable image acquisition.
References
1 Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer
K, Nadeau RG: Orthogonal polarization spectral imaging: a new
method for study of the microcirculation. Nat Med 1999,
5:1209-1212.
2 Ince C: The microcirculation is the motor of sepsis. Crit Care
2005, 9(Suppl 4):S13-S19.
3 De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL:
Microvascular blood flow is altered in patients with sepsis. Am
J Respir Crit Care Med 2002, 166:98-104.
4 Boerma EC, van dV, Spronk PE, Ince C: Relationship between
sublingual and intestinal microcirculatory perfusion in patients
with abdominal sepsis. Crit Care Med 2007, 35:1055-1060.
|