|
Abstract
Demographic changes, i. e. worldwide increase in older
population, require to direct research ef¬forts to age-relevant
topics. The present article focuses on microcirculation in
elderly patients. Sidestream dark field (SDF) imaging(visualize the microcirculation at the bedside,
Cerebral microcirculation,Brain microcirculation,Renal microcirculation,Kidney microcirculation,
Sublingual microcirculation), a relatively new technology, allows direct visualization of mucos¬al microcirculation and imaging of surface layers of solid
organs using a handheld microscopical camera probe. Our study
aimed at assessing sublingual microcirculation in elderly
patients using this new SDF technology (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) in order to identify and
compare changes across different age groups. The study results
suggest that even in healthy individuals microvascular flow
index (MFI) and functional capillary density (FCD) change
significantly during the process of aging.
Key words: microcirculation, sublingual, elderly,
Sidestream dark field (SDF) imaging (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)
Introduction
These changes in the world��s demographics drive focus also of microcirculation��visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation�� The last decades
witnessed an increase in the research towards elderly patients.
An under¬world��s older population, specially in the de-standing
of age-dependent adaptation in the veloped countries, this being
the result of a structure and function of microvascular net¬more
advanced health care, better social sys-works is critical to
understanding how deliv¬tems and improved living conditions in
de-ery and distribution of blood flow is con¬veloped countries.
The U.S. Census Bureau trolled across the life span. released a
report in 2009 that showed that sidestream dark field (SDF) imaging(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)
has risen as the world��s 65-and-older population is pro-a
pioneer among different technologies dejected to triple by mid
century, from 516 mil-signed to image the human microcirculation
lion in 2009 to 1.53 billion in 2050. In con-(1-3). This
relatively new technology allows trast, the population under 15
is expected to the direct visualization of mucosal
microcir¬increase by only 6 percent during the same culation and
imaging of surface layers of sol¬period, from 1.83 billion to
1.93 billion. id organs using a handheld microscopical From 2009
to 2050, the world��s 85 and old-camera probe. This technology
depends on a er population is projected to increase more light
guide imaging the microcirculation, than fivefold, from 40
million to 219 million. which is surrounded by light emitting
diodes
at a wavelength of 530nm. The hemoglobin of the erythrocytes
absorbs this green light while the rest of the light is
scattered to form the white-greyish background. This produces
clear images of capillaries with flowing ery¬throcytes (4). SDF
(visualize the microcirculation at the
bedside,Cerebral microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)has played a role in min¬imizing the gap in understanding the
micro¬circulation and helped move microcirculato¬ry research
from bench to bedside.
The primary aim of our study was to study the sublingual
microcirculation in eld¬erly patients using SDF technology (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation). To iden¬tify changes in microcirculatory parameters during the
physiological process of aging we compared the microcirculation
of different age groups (20-39, 40-69, 75-90 years).
Methods
Subject Selection
Approval for the study was granted by the REB of the University
Hospital, Hradec Kr��lov��, Czech Republic. Written informed
consent was obtained from all participants.
We divided the study subjects in three groups, regardless of
their sex. The first group (Group A, n=10) included healthy
subjects (ages 20-39 years), the second group (Group B, n=10)
included 10 healthy subjects (ages 40-69), and the third group
(Group C, n= 10) healthy subjects (ages 70¬90).
Since the aim was to study the physiolog¬ical changes during
aging, in all groups we included only subjects that were
healthy, did not have any chronic diseases and did not take any
medications. Furthermore, they were all of good physical
condition, with the elderly capable of performing daily
activities on their own without the need of assistance. All
subjects were of good mental health, with no history of dementia
(6), Alzheimers (7), psychosis or behavioral disturbances. All
our subjects were non-smokers (8, 9). All subjects had
hematocrit values within the normal ranges.
We excluded subjects with any previous history of conditions
that could cause patho¬logical changes in the microcirculation(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation).
Our subjects did not have any history of hyper¬tension (12),
ischemic heart disease, diabetes mellitus, ischemic diseases of
the lower ex¬tremities, cerebrovascular diseases, critically ill
patients, subjects undergoing mechanical ventilation or being
treated for all forms of circulatory shock (13-16).
All subjects were instructed to refrain from consuming
caffeine-containing sub¬stances 2 h prior to the evaluation. No
seda¬tion was used during the image recordings and all subjects
were cooperative.
Study settings
Image recordings of the microcirculation (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)took place at two
locations. Subjects from group A and B where invited to the
library of the Department of Anesthesiology and Inten¬sive Care
Medicine, University Hospital of Hradec Kr��lov��. Subjects from
group C where residents of the Senior Home of Hradec Kr��lov��,
and to ensure their comfort, the image recordings where taken at
site at the in-house clinic. Each subject was exam¬ined
individually in the supine position to al¬low comfortable
measuring for both the sub¬jects and the examiners, the elderly
patients were asked to remove any dental prostheses.
The sublingual microcirculation was vi¬sualized using the
MicroScan SDF (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)camera (MicrovisionMedical, Amsterdam, The
Netherlands). In order to facilitate the proce¬dure and minimize
artifacts, all images were obtained by two investigators with
long¬standing experience in this technique. The SDF (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)probe was
covered with a sterile plastic lens and lightly placed on the
target sublin¬gual mucosa, the subjects were asked to hold their
mouth in a semi-closed position in or¬der to help positioning
the camera. Two trained physicians blinded to clinical data
performed measurements. Subjects were in supine position, in a
temperature controlled room with a temperature of approximately
22��C. The tip of the SDF (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) probe was placed
on sublingual mucosa. To prevent microcir¬culatory perfusion
disturbance due to appli¬cation of pressure on the imaging area,
the probe was first placed on the labial tissue and then
retracted to an extent, which mini¬mized contact but enabled
visualisation of the capillary bed. Illumination intensity and
depth of focus were modulated to fine-tune image quality.
Continuous digital image recordings (duration 1 min) were
captured in five different locations under the tongue, and
digital image recordings were saved on a hard drive as DV-AVI
files to enable offline analysis. For high quality image
recording we followed the recommendations of the round table
consensus on image acquisition and analysis (17).
Off-line analysis
SDF (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation)video clips were coded and analysed off line, using the
methods described by De Backer et al. (17). Data were analyzed
using the AVA3 software (MicrovisionMedical, Amesterdam, The
Netherlands). The soft¬ware enables automatic detection of
vessels (7 -100 µm) for the calculation of their diam¬eter,
length, density and flow. We used the software to calculate
Microvascular Flow In¬dex (MFI), and Functional Capillary
Density (FCD).
To calculate the MFI, the image was di¬vided into four
quadrants. Characteristic flow scores were assigned in each
quadrant for each vessel size category. The flow categories are 0
for no flow, 1 for intermittent flow, 2 for sluggish flow and 3 for
continuous flow. The flow category as¬signed to each vessel
category is then summed for the four quadrants and divided by
the number of quadrants in which the ves¬sel type is present (MFI
range 0�C3).
FCD is defined as the length of red blood cell-perfused
capillaries per observation area and is given in cm/cm2. FCD is
calculated by applying three equidistant horizontal and three
equidistant vertical lines superimposed upon the video sequence.
FCD was calculat¬ed as the number of capillaries crossing the
lines divided by their total length. This gave the number of
capillaries per mm. FCD for small vessels was estimated in
vessels with less than 25 µm in diameter.
Statistical analysis
Statistical analysis was performed using Prism 4 (GraphPad, La
Jolla, CA, USA). All data were analyzed using a one-way analysis
of variance (ANOVA), followed by the Tukey post hoc test. A p
value < 0.05 was consid¬ered significant.
Results
Between groups A (20-39) and B (40-69) we did not observe
significant differences in the FCD of all vessels, FCD of small
vessels or MFI (Figures 1-3). However, significant de¬creases in
the FCD of all vessels, FCD of small vessels and MFI was found
when com¬paring groups A (20-39) and C (70-90) or B (40-69) and
C (70-90), respectively.
Discussion
We found a significant decrease in the mi¬crocirculatory
parameters FCD and MFI in group C (70-90) when compared to the
oth¬er two groups A (20-39) and B (40-69). In group C the
significant decrease in FCD was found in small capillaries (less
than 25 µm in diameter) as well in all vessels. The greatest
difference was found in the FCD of small vessels.
The dramatic increase in the number of people reaching age 65 �C
coupled with their increased life expectancy �C has expanded the
classification of those of age 65 and old¬er to include three
sub-populations com¬monly referred to as the „young old�� (being
the age of 65-74), the „old�� (74-84), and the „old-old�� (being
older than 85; (5)). To sim¬plify our study design, we divided
the sub¬jects into three sub-groups (group A: 20-39, group B:
40-69 and Group C: 70-90 years).
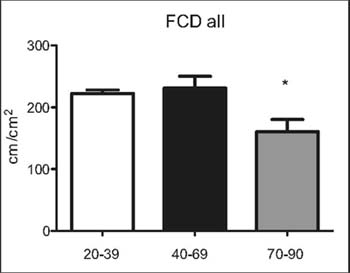
Figure 1: Functional capillary densi-ty (FCD) of all
microvessels (<100µm), n=10 per group.* p<0.05 vs. 20-39 and
40-69 yearsold
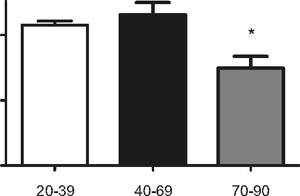
Figure 2: Functional capillary densi-ty (FCD) of small
microvessels (<25µm), n=10 per group.* p<0.05 vs. 20-39 and
40-69 yearsold
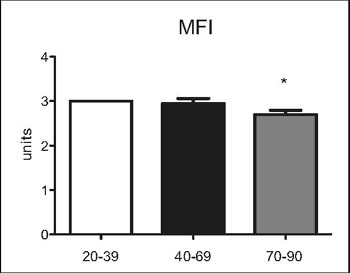
Figure 3: Microvascular Flow Index(MFI) of all microvessels
(<100 µm),n=10 per group.* p<0.05 vs. 20-39 and 40-69 yearsold
��
All subjects had hematocrit values within the normal
ranges. The hematocrit in the prefer¬ential flow channels is an
inverse function of the flow rate for any level of the
microcircu¬latory hematocrit. The increased hematocrit raises
the flow resistance in these vessels which reduces flow further
and represents a positive feedback condition which may
con¬tribute to the intermittent and uneven flow patterns which
are present within the micro¬circulation (10, 11)
Our results concur with previous results from studies that
looked at the microcirculatory(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) changes during the process of
aging. Ex¬perimental data indicate that, independent of the
presence of other pathologies, aging al¬ters
endothelium-dependent relaxations in both the aorta and small
resistance arteries in rats (18-21). Brandes et al. (22)
suggested that aging is associated with a reduction in the
regenerative capacity of the endothelium and endothelial
senescence, which is char¬acterized by an increased rate of
endothelial cell apoptosis. Muller et al. (23) showed that aging
impairs endothelium-dependent va¬sodilation in rat skeletal
muscle arterioles.
Taddei et al. (24) evaluated the role of ad¬vancing age as an
independent factor that can alter endothelial function. They
demon¬strated that the vasodilating response to acetylcholine
decreased with advancing age in the forearm of both normotensive
control subjects and essential hypertensive patients, whereas
the vasodilating response to sodium nitroprusside was minimally
affected by ag¬ing. Taken together, these results are
consis¬tent with the finding that endothelial func¬tion is
progressively impaired with aging (24). In a separate study the
same group pro¬posed the age related endothelial dysfunc¬tion to
be mediated by a progressive reduc¬tion of NO availability,
since the inhibiting effect of L-NMMA on acetylcholine-induced
vasodilation was progressively impaired by advancing age (25).
Angula et al. (26) showed that although the aging process by
it¬self, without other concomitant morbidities, causes an
impairment of endothelium-de¬pendent vasodilation of human
vessels, the
presence of cardiovascular risk factors exac¬erbated such
impairment in aged human ves¬sels. Furthermore they suggested
that the re¬sponse of aged human vessels to treatments for
improving (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) endothelial function might be different from that of
vessels from adult sub¬jects (26). Eskurza et al. (27) confirmed
that oxidative stress was the main mediator of the age related
endothelial dysfunction.
A group of studies focused on evaluating the blood flow in
specific muscle groups dur¬ing exercise. Wahren et al. (28)
showed that the rise in leg blood flow during exercise was
decreased in older male subjects (52�C59 years) compared to the
values measured in young male subjects (25�C30 years). Proctor et
al. (29) have also shown that leg blood flow and vascular
conductance during sub¬maximal cycling exercise at a given level
of whole-body oxygen consumption are sub¬stantially reduced in
older men as compared to their young counterparts. Furthermore,
muscle blood flow is lower in older human subjects when a small
muscle mass is active and the limits of cardiac output are not
ap¬proached (30). In conclusion, data obtained in humans
indicate that age-induced adapta¬tions of the vasculature
contribute to a reduc¬tion in muscle blood flow; however, the
spe¬cific mechanisms that contribute to the age¬related
diminution of blood flow to muscle have not been discerned in
human models (31).
The affect of aging on the density of mi¬crovessels has not been
studied in detail yet, and there is no striking consensus on the
ef¬fects of aging on capillary density. Hutchins et al. (32) and
Sonntag et al. (33) demonstrat¬ed a substantial aging-related
rarefaction of the surface arterioles that supply the
parenchymal vessels of the cerebral cortex in rats. In normally
aging rats, the density of ar¬terioles was almost 40% lower in
senescent animals than in young adults (29 months ver¬sus 7
months of age).
SDF(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) was not previously used for evalua¬tion of the
microcirculation (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) in elderly pa¬tients. Previous studies mainly
focused on the molecular level to illustrate the age relat¬ed
endothelial dysfunction (24-26) or used techniques such as the
constant-rate intra-ar¬terial indicator infusion technique to
study the changes in blood flow (27, 34). To our knowledge no
study has evaluated the capil¬lary density in humans.
Conclusion
We were able to identify in healthy subjects the age group at
which the microcirculatory parameters FCD and MFI change
significant¬ly. Individuals above the age of 70 years should be
excluded from studies evaluating the microcirculation (visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation) because of
the age-relat¬ed changes found in our study. Even though
individuals above the age of 70 years could be free from
pathological conditions, stan¬dard microcirculatory parameters
are altered.
Acknowledgements
Supported by the project (Ministry of Health, Czech Republic)
for conceptual develop¬ment of research organization 00179906
and by the programme PRVOUK P37/02.
References
1 Ince C. The microcirculation is the motor of sepsis. Crit Care
2005; 9 (Suppl. 4): 13¬19
2 Ince C. sidestream dark field (SDF) imaging
(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation): an improved technique
to observe sublingual microcirculation. Crit Care 2005; 9 (Suppl.
1): 72
3 Turek Z, Cerný V, Pař��zkov�� R. Noninva¬sive in vivo assessment
of the skeletal mus¬cle and small intestine serous surface
mi¬crocirculation in rat: sidestream dark field (SDF) imaging
(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation). Physiol Res 2007; 56
4 Goedhart PT, et al. Sidestream Dark Field (SDF) imaging(visualize
the microcirculation at the bedside,Cerebral
microcirculation,Brain
microcirculation,Renal
microcirculation,Kidney
microcirculation,Sublingual
microcirculation): a
novel stroboscopic LED ring-based imaging modality for clinical as¬sessment of the microcirculation. Optics express 2007; 15
(23): 15101-15114
.......etc. |