|
Abstract
Background and PurposeˇŞPlatelet-activating factor (PAF) is
involved in the development of secondary brain (Cerebral microcirculation,
brain microcirculation)damage after ischemic and traumatic brain
injury. On the basis of data from studies in peripheral organs,
we hypothesized that PAF-mediated effects after cerebral (Cerebral microcirculation,
brain microcirculation)injury
could be secondary to alterations in
cerebral microcirculation.
MethodsˇŞChanges in cerebral
microcirculation(brain
microcirculation) focusing on leukocyte-endothelium
interactions were quantified with the use of a closed cranial
window model in Sprague-Dawley rats (n=33) by means of
intravital fluorescence microscopy. The brain(Cerebral microcirculation,
brain microcirculation) surface was superfused with PAF in concentrations from 10−3 (n=3) to 10−12
mol/L (n=6) for 20 minutes (5 mL/h).
ResultsˇŞPAF 10−4 mol/L (n=4) increased the number of rolling and
adherent leukocytes in venules from 9.7ˇŔ0.4 to 19.7ˇŔ2.3
cells/100 mm • min (P=NS versus control) and from 2.2ˇŔ0.5 to
4.3ˇŔ0.7 cells/100 mm • min (P<0.05 versus control),
respectively. Lower concentrations did not elicit
leukocyte-endothelium interactions. Vessel diameters remained
unchanged except for a transient increase of arteriolar
diameters during superfusion with PAF 10−4 and 10−6 mol/L (n=6).
Although only a limited area of the brain(Cerebral microcirculation,
brain microcirculation) surface was exposed to PAF, the mediator induced a significant dose-dependent
transitory arterial hypotension and caused irreversible
circulatory shock at the high concentration (PAF 10−3 mol/L).
Arterial hypotension after administration of PAF 10−3 mol/L
could be attenuated by the intravenous pretreatment with the PAF
antagonist WEB 2170BS.
ConclusionsˇŞPAF, when locally released after brain (Cerebral microcirculation,
brain microcirculation)injury, can
penetrate the blood-brain barrier and induce systemic effects,
including arterial hypotension. Its role as a mediator in the
development of secondary brain damage seems, at least in the
initial phase, not to be associated with disturbances of
cerebral microcirculation (brain microcirculation)or activation of leukocytes.
Key Words: cerebral
circulation,leukocytes,microcirculation(Cerebral microcirculation,
brain microcirculation),platelet activating
factor,rats
Platelet-activating factor (PAF) is an endogenous
phospholipid considered to be an important mediator in
inflammatory and allergic diseases as well as in shock.1 Several
reports have also provided evidence for a role of PAF in
ischemic and traumatic brain (Cerebral microcirculation,
brain microcirculation)injury.2 PAF can be synthesized by
brain cells and is found in small quantities in normal brain
tissue.3 4 Increased levels of PAF were found in experimental
models of ischemia as well as in patients with ischemic stroke.5
6 Furthermore, pretreatment and posttreatment with PAF
antagonists in global and focal ischemia can improve the
survival of neurons and the neurological outcome.7 8 The exact
underlying pathophysiological mechanisms of the various effects
mediated by PAF are still under discussion. Intravital
microscopic studies in peripheral tissues have shown that local
application of PAF increases vascular permeability,9 causes
arteriolar vasoconstriction,10 and induces leukocyte-endothelium
interactions in venules.11 In a previous intravital microscopic
study using a closed cranial window preparation in the rat, we
have observed that the intra-arterial infusion of PAF into the
internal carotid artery in concentrations found during shock
induces leukocyte-endothelium interactions in cerebral(Cerebral microcirculation,
brain microcirculation) venules
independent of changes in microvascular flow or of systemic
parameters such as arterial blood pressure.12 The specificity of
these effects was proven by the pretreatment of the animals with
the PAF receptor antagonist WEB 2170BS, which could inhibit the
PAF-induced decrease in systemic blood pressure as well as the
induction of leukocyte-endothelium interactions. Whether a local
release of PAF would also elicit leukocyte-endothelium
interactions in the cerebral microcirculation
(brain microcirculation)has not been
studied thus far. Therefore, the objective of the present in
vivo study was to assess the effects of locally administered PAF
on cerebral microcirculation(brain microcirculation) and the induction of
leukocyte-endothelium interactions in pial venules.
Materials and Methods
Animals
Thirty-three male Sprague-Dawley rats (body weight, 295ˇŔ23 g) were
used. The laboratory-acclimated animals had free access to tap
water and standard pellet food. The experiments were conducted
according to institutional guidelines and were approved by the
state government of Bavaria.
Surgical Preparation
The surgical preparation has been described in detail previously.12
Briefly, after induction of anesthesia with pentobarbital 3.6%
(10 mL/kg body wt IP), the animals were placed on a
feedback-controlled heating pad (Effenberger) with the rectal
temperature maintained at 37.5ˇŔ0.6ˇăC. Femoral arterial and
venous catheters were surgically inserted for continuous
measurement of arterial blood pressure, blood sampling, and
infusion of anesthetics and fluorescent dyes. After tracheotomy
and intubation, the animals were immobilized with pancuronium
bromide (initial bolus of 1.2 mg/kg body wt IV followed by
continuous infusion of 1.2 mg/h) and mechanically ventilated
(Harvard ventilator model 683; PaCO2, 36 to 40 mm Hg; PaO2, 100
to 120 mm Hg). Anesthesia was continued by intravenous ¦Á-chloralose
(Merck; bolus of 5 mg/kg body wt). Arterial blood samples (0.1
mL) were obtained in 20-minute intervals for measurement of
arterial blood gases, pH, base excess, and hemoglobin
concentration (ABL 300, Radiometer A/S). In cases of a negative
base excess >10 mmol/L, animals received NaHCO3.12 Systemic
leukocyte count and hematocrit were assessed immediately after
implantation of the catheters; before (0 minutes), during (10
minutes), and at the end of the PAF application (20 minutes);
and at the end of the experiment (120 minutes). The mean
arterial blood pressure, intracranial pressure, and airway
pressure were continuously monitored (Honeywell, model 3260
recorder). After fixation of the skull in a stereotaxic frame
(model 900, David Knopf Inc), a closed cranial window for
intravital microscopy equipped with an inflow and outflow
catheter for superfusion of the brain (Cerebral microcirculation,
brain microcirculation)and monitoring of
intracranial pressure was implanted over the left parietal brain(Cerebral microcirculation,
brain microcirculation)
hemisphere, as described.12
Platelet Activating Factor and WEB 2170BS
PAF (C-16 PAF;
1-O-hexadecyl-2-(R)acetyl-sn-glycero-3-phosphocholine; molecular
weight, 525.7 Da; Bachem AG) dissolved in artificial
cerebrospinal fluid containing endotoxin-free bovine serum
albumin (0.5% solution; Sigma) was administered in
concentrations of 10−12, 10−9, 10−6, 10−4, and 10−3 mol/L. With
the use of a superfusion rate of 5 mL/h for 20 minutes, total
doses from 0.88 pg (10−12 mol/L; 44 fg/min) to 0.88 mg (10−3
mol/L; 44 ¦Ěg/min) were superfused. Control animals were
superfused with mock cerebrospinal fluid without PAF. Because of
the known tachyphylaxis of PAF, only one concentration was
administered in each individual animal. For specific inhibition
of the effects induced by PAF, the competitive PAF receptor
antagonist WEB 2170BS (provided by Boehringer Ingelheim) was
used.13 The antagonist (2 mg/kg body wt) was dissolved in
isotonic saline and injected intravenously 15 minutes before
superfusion of the brain (Cerebral microcirculation,
brain microcirculation) with PAF 10−3 mol/L. The latter
concentration was chosen because it was found to elicit the most
deleterious effects. In 3 animals, PAF concentrations in blood
were analyzed after superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©with PAF 10−6 mol/L
with the use of a commercially available standardized
125I-labeled PAF radioimmunoassay (DuPont de Nemours GmbH, NEN
Division). Blood samples of 1 mL were taken before superfusion
with PAF (0 minutes), at the end of superfusion with PAF (20
minutes), and at the termination of the experiment (120
minutes). These animals were not subjected to intravital
microscopy.
Intravital Fluorescence Microscopy
The workstation for intravital fluorescence microscopy has been
described in detail previously.12 Before each measurement,
leukocytes were labeled in vivo by intravenous injection of 0.1
mL of a 0.1% rhodamine 6G solution (Sigma Chemical). The
intravital microscopic images were recorded by a video camera
and stored on videotapes for offline evaluation, as reported.
The integrity of the blood-brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©barrier (BBB) was studied
online at the end of the experiment by the intravenous injection
of 0.5 mL of a 5% Na+-fluorescein solution (Sigma Chemical).
Analysis of Microcirculatory Parameters
The measurements included arteriolar and venular diameters
(micrometers), the number of rolling or adherent leukocytes in
venular vessel segments of 100-¦Ěm length during an observation
period of 1 minute (cells/100 ¦Ěm • min), and the opening of the
BBB (yes/no). Diameters were measured with the use of a
computer-assisted analysis system (CAMAS).14 In each venular
vessel segment, the velocities (millimeters per second) of at
least 30 leukocytes freely moving in the central flow axis were
measured at each time point, and the harmonic mean was
calculated. The result served as an estimate of the blood flow
velocity in venules. Using the diameters of the venules (D) and
the velocity of freely moving leukocytes (V), we calculated the
shear rate ¦Ă (per second) according to the formula ¦Ă=(Vmean/D)ˇÁ8.
Experimental Design
After implantation of the closed cranial window and start of
superfusion with mock cerebrospinal fluid, the animals were
allowed to stabilize during a control period of 60 minutes.
During this period, 4 control measurements were performed at
20-minute intervals. Before the first measurement, 2 to 3
regions of interest with at least 1 pial arteriole and 1 pial
venule were selected. Thus, in each animal at least 2 to 3
arterioles and 2 to 3 venules were observed and analyzed. After
the control period, the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©was superfused with PAF for 20
minutes. Further intravital microscopic observations were
performed 5 minutes after the start and at the end of the
superfusion (20 minutes) of PAF or the vehicle, then every 20
minutes for 2 hours. At the end of the experiment, the integrity
of the BBB was investigated by the intravenous injection of Na+-fluorescein.
Six animals were randomly assigned to the different groups with
the superfusion of PAF at concentrations of 10−12 to 10−4 mol/L.
All animals exposed to PAF 10−3 mol/L experienced irreversible
circulatory shock. Therefore, the number of animals in this
group was limited to 3. Since PAF at a concentration of 10−3
mol/L had the most deleterious systemic effects, another 3
animals received WEB 2170BS (2 mg/kg body wt IV) 15 minutes
before superfusion with PAF.
Data Analysis
Statistical analysis was performed with SigmaStat 1.0 software (Jandel
Inc). Because of the limited number of animals, nonparametric
distribution of the data was assumed. Therefore, the Kruskal-Wallis
test, followed by the Mann-Whitney U test, together with the
Bonferroni-Holm procedure for repeated measurements was used to
analyze differences between control and treated groups.
Statistical significance was assumed at P<0.05. All values are
reported as meanˇŔSD.
Results
Systemic Parameters
PAF induced a dose-dependent decrease of mean arterial pressure
leading to an irreversible hypotensive shock after superfusion
with PAF 10−3 mol/L in all animals (Figure 1). These animals
were not included in the evaluation of the microcirculatory
parameters. The hypotension induced by PAF 10−3 mol/L could be
partially inhibited by pretreatment of the animals with the
specific PAF receptor antagonist WEB 2170BS (Figure 1). PAF 10−4
and 10−6 mol/L induced a transient decrease of arterial blood
pressure, which recovered after the superfusion was discontinued
(Figure 1). Two animals superfused with PAF 10−4 mol/L also
experienced irreversible circulatory shock and were excluded
from microcirculatory analysis. The lower concentrations (PAF
10−9 and 10−12 mol/L) had no effect on blood pressure (data not
shown). Furthermore, superfusion with PAF at a concentration of
10−4 mol/L led to acidosis: pH dropped from 7.37ˇŔ0.04 to
7.23ˇŔ0.08 within 60 minutes, and base excess decreased by 9.2
mmol/L. The lower concentrations had no influence on base excess
or pH. Intracranial and airway pressure, hemoglobin, hematocrit,
and the systemic leukocyte count remained constant in all groups
and did not differ from the control group (data not shown).
After superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface with PAF 10−6 mol/L, PAF
concentration in blood increased from 8.5ˇŔ2.5 to 16.6ˇŔ7.7 ng/mL
20 minutes after start of the superfusion. The PAF concentration
in blood returned to baseline at the end of the experiment
(9.2ˇŔ3.4 ng/mL).
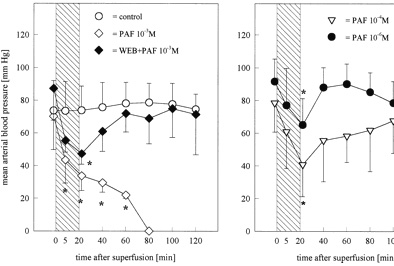
Figure 1.
Mean arterial blood pressure before, during, and after
superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©(for 20 minutes) with PAF at
concentrations of 10−3 (n=3), 10−4 (n=4), or 10−6 mol/L (n=6)
and animals pretreated with WEB 2170BS (2 mg/kg body wt IV; n=3)
15 minutes before the application of PAF in a concentration of
10−3 mol/L. Values are meanˇŔSD. *P<0.05 vs control, Mann-Whitney
U test with Bonferroni-Holm correction. Superfusion with PAF was
followed by a dose-dependent decrease of mean arterial blood
pressure. Data of PAF 10−9 and 10−12 mol/L are not shown because
no effects on arterial blood pressure were observed. Bar
indicates time of PAF infusion.
Microcirculatory Parameters
Whereas the venular diameters did not change throughout the
experiment (data not shown), a dilation of arterioles was
observed during the superfusion with PAF 10−4 and 10-6 mol/L
(Figure 2). This was found to be coincident with reduction of
the mean arterial pressure. Lower concentrations of PAF had no
effect on the arteriolar diameters (Figure 2). The number of
rolling or adherent leukocytes did not change in either the
control group or in animals superfused with PAF in
concentrations from 10−6 to 10−12 mol/L (Figures 3 and 4). A
significant increase (P<0.05 versus control) in the number of
adherent leukocytes from 2.2ˇŔ0.5 to 4.3ˇŔ0.7 cells/100 mm • min
was found 60 minutes after superfusion with PAF 10−4 mol/L
(Figure 4). The number of rolling leukocytes increased from
9.7ˇŔ0.4 to 19.7ˇŔ2.3 cells/100 mm • min without reaching
significance compared with control (Figure 3). Leukocyte
velocity (data not shown) and subsequently the shear rate in
venules (Figure 5) were significantly reduced (P<0.05) within 40
minutes after the application of PAF 10−4 mol/L and remained low
throughout the experiment. In all other groups, neither
parameter changed significantly. No extravasation of Na+-fluorescein
at the end of the experiment was observed, indicating complete
integrity of the BBB (Figure 6a). Opening of the BBB was only
observed in the animals superfused with PAF 10−3 mol/L (Figure
6b) and could not be prevented by pretreatment with the PAF
antagonist.
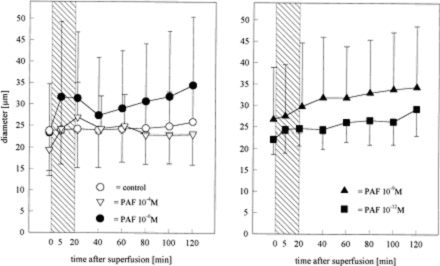
Figure 2.
Diameters of pial arterioles before, during, and after
superfusion of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ© with PAF at concentrations of 10−4
(n=4), 10−6 (n=6), 10−9 (n=6), or 10−12 mol/L (n=6). Values are
meanˇŔSD. During superfusion, vessel diameters increased in the
group treated with PAF 10−4 and 10−6 mol/L (P=NS vs control).
Bar indicates time of PAF infusion.
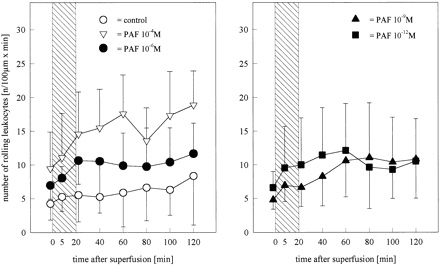
Figure 3.
Number of leukocytes rolling along the luminal surface of pial
venules before, during, and after superfusion of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ© with PAF at concentrations of 10−4 (n=4), 10−6 (n=6), 10−9 (n=6), or
10−12 mol/L (n=6). Values are meanˇŔSD. Although it was not
statistically significant, an increase in the number of rolling
leukocytes was observed in the group superfused with PAF 10−4
mol/L. Bar indicates time of PAF infusion.
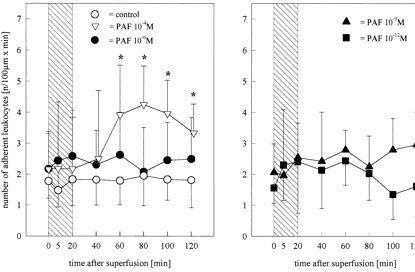
Figure 4.
Number of leukocytes attached to the endothelium of pial venules
before, during, and after superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface with PAF at concentrations of 10−4 (n=4), 10−6 (n=6), 10−9 (n=6), or
10−12 mol/L (n=6). Values are meanˇŔSD. *P<0.05 vs control,
Mann-Whitney U test with Bonferroni-Holm correction. A
significant increase in the number of adherent leukocytes was
observed in the animals superfused with PAF 10−4 mol/L. Bar
indicates time of PAF infusion.
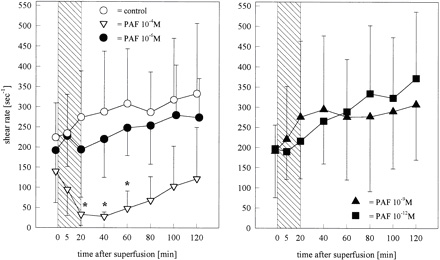
Figure 5.
Shear rate in pial venules calculated on the base of leukocyte
velocities and venular diameters before, during, and after
superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©with PAF at concentrations of 10−4
(n=4), 10−6 (n=6), 10−9 (n=6), or 10−12 mol/L (n=6). Values are
meanˇŔSD. After superfusion with PAF 10−4 mol/L, the shear rate
decreased significantly and did not return to baseline values
until the end of the experiment. Bar indicates time of PAF
infusion.
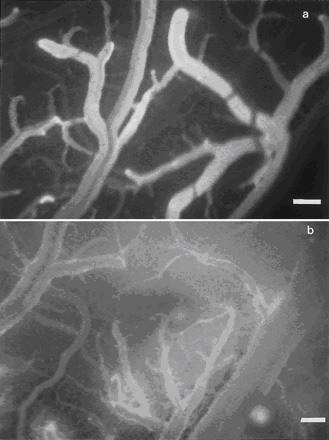
Figure 6.
a, Fluorescence microphotographic view of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface with
contrast enhancement of microvessels by injection of 0.5 mL 5%
Na+-fluorescein intravenously at the end of the experiment, 2
hours after start of the superfusion with PAF 10−6 mol/L. The
absence of the extravasation of the plasma marker indicates an
intact BBB. b, Extravasation of Na+-fluorescein after
superfusion of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ© with PAF 10−3 mol/L, demonstrating the
increased permeability of the BBB. Bar=80 ¦Ěm.
Discussion
Systemic Parameters
Arterial hypotension was the most striking response to
superfusion of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ© surface with PAF. Thus far, however,
this hypotensive effect has only been described after systemic
application of the phospholipid.13 15 It is nevertheless
surprising that the exposure of only a limited area of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ©
surface to PAF resulted in a significant dose-dependent and
transient systemic hypotension. At high concentrations (10−3
mol/L), circulatory shock was irreversible. Lower concentrations
(PAF 10−9 and 10−12 mol/L) had no effect on arterial blood
pressure. A similar observation was reported in 1988 by Armstead
et al,16 who superfused the brain surface of newborn pigs with
up to 0.1 ¦Ěg of PAF, a dose that corresponds to the
concentration of 10−9 mol/L in our study. The blood pressure of
the pigs remained unaffected. The systemic hypotension after
local cerebral (Cerebral microcirculation,
brain microcirculation)application does not seem to be mediated by local
receptors of cerebral (Cerebral microcirculation,
brain microcirculation)blood vessels but may be attributed to a
secondary increase in systemic levels of vasoactive mediators17
18 and a direct relaxant effect of PAF on arteriolar smooth
muscle cells in the peripheral circulation, with a consecutive
reduction of systemic vascular resistance.19 This conclusion is
supported by findings following the pretreatment with the PAF
antagonist WEB 2170BS, which could at least partially inhibit
the hypotension after superfusion with PAF 10−3 mol/L.
With the exception of decreases in pH and base excess, no
changes in systemic parameters, including intracranial pressure,
airway pressure, hemoglobin concentration, hematocrit, or
systemic leukocyte count, were found, in contrast to
observations in the same model after intra-arterial injection of
PAF.12 We believe that the superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©did not
result in systemic concentrations of PAF high enough to cause
the changes observed after intra-arterial application. The PAF
concentration in blood 20 minutes after the start of superfusion
reached only 29% (16.6ˇŔ13.3 ng/mL) of the concentration found
after intra-arterial infusion (56.8ˇŔ22.9 ng/mL).12
Vessel Diameters
In contrast to studies in peripheral organs, local superfusion
of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface with PAF did not result in a significant
change of vessel diameters. The increase in arteriolar diameters
during the superfusion with PAF 10−4 mol/L and PAF 10−6 mol/L
may be attributed to an autoregulatory reaction to the reduction
of systemic blood pressure rather than to a direct local
dilatory effect of PAF. A similar observation was made after the
intra-arterial infusion of PAF into the carotid artery.12
Furthermore, PAF concentrations from 10−12 to 10−6 mol/L did not
change diameters of cerebral (Cerebral microcirculation,
brain microcirculation)arterioles of rats in vitro.20
These results are in contrast to studies with isolated feline
and human pial arteries21 : high concentrations of PAF induced
vasoconstriction, whereas the low concentrations (10−7, 10−6
mol/L) induced vasodilatation of prostaglandin F2¦Á¨Cpretreated
arterioles. In newborn pigs, superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface
with PAF was followed by vasoconstriction of pial arterioles.16
The reason for these discrepant findings remains unclear. It is,
however, well known that the microvascular effects of PAF may
vary considerably between species and also between different
tissues in one species.22 23
Blood-Brain Barrier
Although Kumar et al24 have reported that PAF increases the
permeability of the BBB, effects of PAF on BBB integrity remain
controversial. In peripheral organs, superfusion of PAF was
found to increase vascular permeability.9 25 In the present
study, extravasation of Na+-fluorescein was only observed after
superfusion with PAF in an extremely high concentration (10−3
mol/L). Although it inhibited systemic hypotension, supposedly
by blocking PAF receptors of peripheral vessels, pretreatment
with the PAF receptor antagonist did not prevent opening of the
BBB. These findings indicate that the opening of the BBB in our
study seems to be due to a direct detergent-like effect of the
high concentration on the cell membranes and not to a PAF-mediated
morphological change of endothelial cells,26 27 since PAF in a
dose >4 ¦Ěmol can cause disintegration of the lipid bilayers of
cell membranes.28 In contrast to other published data,24 the
increased concentration of PAF in blood after PAF superfusion
indicates that PAF may penetrate the BBB, although it cannot be
completely excluded that some of the mediator reaches the
systemic circulation via the arachnoid granulations. Additional
indirect evidence is derived from the hypotensive response,
which may be blocked by the intravenous injection of the PAF
antagonist during local superfusion of the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©surface with PAF.
Leukocyte-Endothelium Interactions
In recent years, increasing evidence has been provided that
leukocytes may play a crucial role in the development of
secondary brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©damage after ischemia or traumatic brain
injury.29 Leukocyte depletion or blocking of leukocyte adhesion
receptors was found to reduce secondary brain damage and to
improve the functional outcome.30 31 Studies with PAF
antagonists have indicated a role of PAF in the activation and
accumulation of leukocytes after cerebral (Cerebral microcirculation,
brain microcirculation)injury.32 In
peripheral organs, PAF induces margination and adhesion of
leukocytes in postcapillary venules,11 which has been considered
to be the initial step in the inflammatory reaction.33 In
contrast, a marked effect of PAF on leukocyte-endothelium
interactions in the brain could not be detected in the present
studies. Except after superfusion with PAF 10−4 mol/L, an
increase in the number of rolling or adherent leukocytes was not
observed. Concentrations leading to leukocyte-endothelium
interactions in cerebral (Cerebral microcirculation,
brain microcirculation)venules after infusion of the mediator
into the internal carotid artery12 were not effective when
superfused onto the brain. The reason for these discrepant
results is unclear. For activation of leukocyte-endothelium
interactions, leukocytes as well as endothelial cells must be
exposed to PAF. The fact that during superfusion of the brain
the endothelial cells are initially exposed to the mediator with
their abluminal side might have prevented PAF-initiated
presentation of adhesion molecules on these cells, in contrast
to the primary intraluminal contact between PAF and the
endothelial cells on intra-arterial infusion. Alternatively, it
may be hypothesized that during superfusion of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ©,
intravascular levels of PAF were not sufficient to elicit
leukocyte-endothelium interactions because of the rapid
inactivation of the substance by the brain Ł¨Cerebral microcirculation,
brain microcirculationŁ©acetyl-hydrolase34
together with inhibition of diffusion by the BBB. The present
findings, however, do not exclude that continuous release of PAF
after brain injury can induce leukocyte-endothelium
interactions. Indeed, blood levels of PAF were found to be
increased in patients with ischemic stroke.6 Furthermore, since
our experiments have been performed with an intact BBB, a
primary disruption of the BBB after ischemia or trauma may
likely facilitate leukocyte-endothelium interactions from a
local release of PAF in the brain.
In summary, in contrast to the systemic administration of PAF
and in contrast to respective effects in peripheral organs,
local superfusion of the brain with PAF neither affects the
cerebral microcirculation (brain microcirculation)nor induces leukocyte-endothelium
interactions, except in extremely high concentrations that cause
irreversible circulatory shock. Therefore, the present findings
do not support the hypothesis that the release of PAF in the
early phase after cerebral (Cerebral microcirculation,
brain microcirculation)injury contributes to the activation
of leukocytes or the impairment of cerebral microcirculation.
Nevertheless, it cannot be ruled out that rolling, adherence,
and emigration of leukocytes together with changes in the microvascular perfusion occur in case of BBB disruption and
continuous release of PAF. Furthermore, the dose-dependent
hypotensive response after the superfusion of only a small area
of the brainŁ¨Cerebral microcirculation,
brain microcirculationŁ© surface indicates that PAF penetrates the BBB.
Local release of PAF at high concentrations in ischemic or
traumatic brain injury could have deleterious effects on
systemic parameters such as arterial blood pressure.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft
Sch¨ą 754-1/1.
Received July 23, 1998.
Revision received October 28, 1998.
Accepted November 30, 1998.
Copyright © 1999 by American Heart Association
References
1.Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in
platelet-activating factor research. Pharmacol Rev.
1987;39:97¨C145.
2.Yue TL, Feuerstein GZ. Platelet-activating factor: a putative
neuromodulator and mediator in the pathophysiology of brain
injury. Crit Rev Neurobiol. 1994;8:11¨C24.
3.Tokumura A, Kamiyasu K, Takauchi K, Tsukatani H. Evidence for
existence of various homologues and analogues of platelet
activating factor in a lipid extract of bovine brain. Biochem
Biophys Res Commun. 1987;145:415¨C425.
http://stroke.ahajournals.org/content/30/4/880.long |