|
Abstract
Purinoceptors are rapidly becoming recognised as important
regulators of tissue and organ function. Renal (Renal microcirculation,kidney microcirculation)expression of P2
receptors is broad and diverse, as reflected by the fact that P2
receptors have been identified in virtually every major
tubular/vascular element. While P2 receptor expression by these
renal structures is recognised, the physiological functions that
they serve remains to be clarified. Renal (Renal microcirculation,kidney microcirculation)vascular P2 receptor
expression is complex and poorly understood. Evidence suggests
that different complements of P2 receptors are expressed by
individual renal vascular segments. This unique distribution has
given rise to the postulate that P2 receptors are important for
renal (Renal microcirculation,kidney microcirculation)vascular function, including regulation of preglomerular
resistance and autoregulatory behaviour. More recent studies
have also uncovered evidence that hypertension reduces renal
(Renal microcirculation,kidney microcirculation)vascular reactivity to P2 receptor stimulation in concert with
compromised autoregulatory capability. This review will
consolidate findings related to the role of P2 receptors in
regulating renal microvascular (Renal microcirculation,
kidney microcirculation)function and will present areas
of controversy related to the respective roles of ATP and
adenosine in autoregulatory resistance adjustments.
Keywords: Afferent arteriole, Autoregulation, Adenosine,
P2X receptors, Hypertension, Tubuloglomerular feedback
Introduction
Understanding the physiology of renal purinoceptors has become a
rapidly developing area of investigation. It is now recognised
that extracellular ATP and adenosine are important signalling
molecules regulating the renal microcirculation(kidney microcirculation) and tubular
function and influencing renin secretion [1¨C20]. This review
will summarise information defining the roles of extracellular
ATP in regulating renal microvascular(Renal microcirculation,kidney microcirculation) function and renal
haemodynamics.
P2 receptors and their expression in the
renal vasculature and glomeruli
P2 receptors are expressed by renal (Renal microcirculation,kidney microcirculation)vascular, glomerular,
mesangial and tubular epithelial cells [1¨C15]. P2X1 receptors
are expressed by vascular smooth muscle of arcuate and
interlobular arteries and afferent arterioles but do not appear
to be expressed by glomeruli or efferent arterioles [4, 12, 21].
P2X1 receptor expression by afferent arteriole vascular smooth
muscle has been confirmed by immunostaining and by western blot
analysis [4, 11]. P2Y1 receptors are found on afferent and
efferent arterioles and P2X2 receptor expression has been
detected in larger intrarenal arteries and veins [6]. P2Y1, P2Y2
and P2X7 receptors are expressed in rat mesangial cells [22, 23]
and P2Y2 receptors are found in podocytes [3, 6]. Mouse
mesangial cells express P2X2, P2X4, P2X7, P2Y2 and P2Y4
receptors. mRNA is detected for P2X1 and P2X3 receptors in
murine mesangial cells but protein is not found by western blot
analysis [9]. Considering the wide distribution of renal P2
receptor subtypes, it seems likely that purinoceptors play
diverse roles in regulating renal vascular and tubular function.
Purinoceptors and renal haemodynamics
The modulation of renal (Renal microcirculation,kidney microcirculation)vascular resistance by nucleotides
and nucleosides has been recognised since the 1920s, but only
received widespread attention in recent decades [24¨C42]. The
renal vascular response to infused ATP or other P2 agonists
depends on many factors including the species, the type of
agonist infused, ambient vascular tone and the experimental
conditions. Intrarenal infusion of ATP into canine kidneys
produces vasodilation by stimulating endothelial release of
nitric oxide [43, 44]. Vasoconstriction is observed in isolated
perfused rat kidneys under basal tone, and either
vasoconstriction or vasodilation when renal (Renal microcirculation,kidney microcirculation)vascular resistance
is elevated [45¨C48]. Infusion of ATP or ¦Á ¦Â-methylene ATP (an
effective P2X1 receptor agonist, which also has some influence
on P2X3 and P2X5 receptors) into the isolated perfused rat
kidney induces a sustained concentration-dependent
vasoconstriction under normal conditions [45¨C49], but
ATP-mediated responses are less consistent under high-tone
conditions [45, 48].
Suprarenal aortic infusion of ATP into anaesthetised rats that
had been fed on a low salt diet led to nitric oxide-dependent
vasodilation in the medullary circulation, whereas in rats fed a
high salt diet, medullary blood flow decreased in response to
ATP [50]. P2Y receptor agonists, such as 2-methylthio ATP, UTP
or ATP-¦Ă-S, produce nitric oxide-dependent vasodilation when
infused at low concentrations but vasoconstriction at higher
concentrations [45, 49]. Thus, endothelial P2Y receptors may
mediate vasodilation due to nitric oxide-dependent relaxation of
the renal microvasculature(Renal microcirculation,kidney microcirculation), which reverts to a pronounced
vasoconstriction during inhibition of nitric oxide synthase
[45]. P2X receptor-mediated vasoconstrictor responses are also
augmented by inhibition of nitric oxide production [45].
P2 receptors and the renal
microcirculation: single vessel studies
Extracellular ATP is an important autocrine and paracrine
regulator of preglomerular vascular responses, mediated by P2X
and P2Y receptor activation [21, 33, 41, 51¨C55]. Infusion of
relatively low doses of ¦Á ¦Â-methylene ATP into the rabbit renal
(Renal microcirculation,kidney microcirculation)artery produces renal cortical and medullary vasoconstriction as
indicated by decreases in regional blood flow [56, 57]. Exposure
of microperfused rabbit afferent arterioles (with attached
glomeruli) to ATP or ¦Â ¦Ă-methylene ATP reduced arteriolar
diameter [41]. Afferent arterioles also express
adenosine-sensitive A1 receptors, raising the question of
whether the vasoconstriction is induced by ATP-dependent
activation of P2 receptors, or whether the ATP is hydrolyzed to
adenosine which vasoconstricts arterioles by activating A1
receptors. The fact that ATP vasoconstricts afferent arterioles
directly was established by demonstrating that ATP-mediated
vasoconstriction persists even during adenosine receptor
blockade and that the sustained vasoconstriction to ATP is
eliminated during P2 receptor blockade [21, 33, 51].
Renal(Renal microcirculation,kidney microcirculation) vascular resistance is regulated primarily by adjusting
afferent arteriolar resistance, with only lesser resistance
contributions being made by the upstream arterial segments [58].
Of the three preglomerular vascular segments evaluated for
responsiveness to P2 receptor activation by ATP, only the
afferent arteriole exhibits sustained ATP-mediated
vasoconstriction at concentrations as low as 100 nM (Fig. 1)
[21]. Arcuate arteries respond to 10 and 100 µM ATP with a
transient vasoconstriction that quickly subsides [21].
Interlobular arteries respond to 100 µM ATP with a sustained
vasoconstriction, but lower concentrations produce no
significant response [21]. Importantly, efferent arterioles are
completely unresponsive to ATP concentrations as high as 100 µM
[21]. These observations support the hypothesis that
extracellular ATP could serve as a paracrine regulator whose
actions primarily influence afferent arteriolar vascular smooth
muscle and thus arteriolar diameter [8, 12, 55, 59].
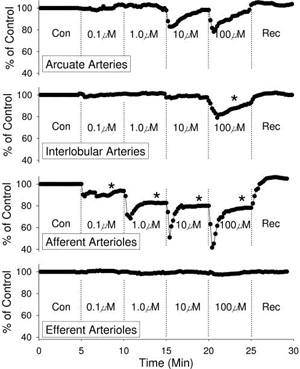 Fig.
1 Fig.
1
ATP concentration¨Cresponse relationship for the intrarenal pre-
and postglomerular vascular segments. Average segmental diameter
responses evoked by ATP applied to the adventitial surface of
arcuate arteries (top panel), interlobular arteries ...
As indicated above, P2Y receptors modulate vascular resistance
by stimulating the synthesis and release of endothelium-derived
relaxing factors [45, 49, 60]. Direct assessment of the
contribution of endogenous nitric oxide in single intrarenal (Renal microcirculation,kidney microcirculation)arteries reveals that the transient ATP-mediated
vasoconstriction of rat arcuate arteries reverts to a sustained
vasoconstriction in kidneys pretreated with l-NAME [60]. This
observation is consistent with whole-kidney studies where
inhibition of nitric oxide synthase with L-NAME greatly reduces
the renal (Renal microcirculation,kidney microcirculation)vasodilation evoked by ATP or P2Y receptor agonists
such as 2-methylthio ATP or UTP [46, 61]. Futhermore, intrarenal
infusion of ATP into the canine kidney in vivo produces a rapid
vasodilation under basal conditions; whereas during inhibition
of nitric oxide synthase, ATP produces sustained renal
vasoconstriction [43]. Thus, nitric oxide is responsible for P2
receptor-mediated renal vasodilation, suggesting that regulation
of renal vascular tone by ATP may involve a complex interplay
between segmental vasoconstrictor and vasodilatory signals
arising from selective, paracrine activation of P2 receptors on
renal vascular smooth muscle cells and endothelial cells. This
idea is supported by observations in other renal (Renal microcirculation,kidney microcirculation)and nonrenal
vascular beds demonstrating that nitric oxide and
endothelium-derived hyperpolarizing factor(s) contribute to the
vasodilation produced by P2Y receptor activation [61¨C66].
P2 receptor-mediated second messenger
systems in the renal microcirculation
Preglomerular vascular smooth muscle cells and glomerular
mesangial cells respond to ¦Á ¦Â-methylene ATP, or ATP, with a
rapid, biphasic increase in intracellular calcium concentration.
This calcium response involves calcium release from
intracellular stores and/or influx of calcium from the
extracellular fluid (see example traces shown in Fig. 2 and Fig.
5b) [9, 11, 59, 67¨C74]. Low concentrations of ATP (ˇÜ1.0 µM)
vasoconstrict afferent arterioles by activation of L-type Ca2+
channels. Higher concentrations of ATP (>1.0 µM) vasoconstrict
afferent arterioles by combining calcium influx and calcium
release to increase the intracellular calcium concentration [11,
59, 73¨C76]. Calcium influx, and afferent arteriolar
vasoconstriction, induced by ATP is markedly attenuated by
blockade of voltage-gated L-type Ca2+ channels [59, 72¨C76].
Removal of extracellular calcium merely blunts the peak increase
in calcium induced by 10 µM ATP, while the sustained elevation
of calcium is abolished (Fig. 2b vs. d) [59, 73, 74]. Thus,
release of calcium from intracellular stores is a major
contributor to the peak calcium response to ATP, while calcium
influx supports the sustained increase in intracellular calcium
concentration [59, 72¨C76]. Interestingly, the sustained
concentration-dependent afferent arteriolar vasoconstriction by
ATP is attenuated, or eliminated, by superfusion with
calcium-free medium or blockade of L-type Ca2+ channels [75,
76].
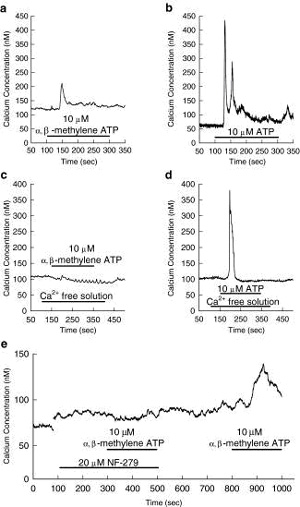 Fig.
2 Fig.
2
The effect of ¦Á ¦Â-methylene ATP and ATP on intracellular calcium
concentration in preglomerular smooth muscle cells. Response of
intracellular calcium concentration evoked by ¦Á ¦Â-methylene ATP
or ATP (each 10 µM) ...
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
ˇˇ
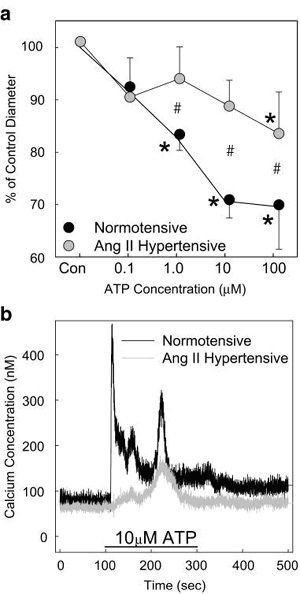 Fig. 5
Fig. 5
Effect of ANG-II hypertension on the afferent arteriolar
diameter and calcium signalling responses to ATP. Panel a
presents the changes in afferent arteriolar diameter in kidneys
from normotensive rats (black symbols) and from Ang-II
hypertensive rats ...
P2X receptors are ligand-gated channels that allow influx of
extracellular Ca2+ and Na+ ions. Activation of P2X receptors on
afferent arterioles leads to calcium influx, whereas calcium
mobilisation from intracellular stores occurs in response to P2Y
receptor activation (Fig. 2) [59, 74]. Thus, P2X1
receptor-mediated vasoconstriction and calcium signalling
responses are eliminated in calcium-free bathing solutions (Fig.
2a vs. c) and during blockade of voltage-gated, L-type Ca2+
channels [59, 72¨C76]. In contrast, P2Y receptor-mediated
vasoconstriction and calcium signalling responses persist during
calcium channel blockade or in calcium-free conditions (Fig.
2d), indicating differing signalling mechanisms between the two
receptor families [59, 72¨C76]. In summary, activation of
voltage-dependent calcium channels and calcium influx are
important signalling elements for ATP-mediated vasoconstriction
of afferent arterioles through P2X1 receptor activation, whereas
calcium release mechanisms predominate in vascular responses
evoked by P2Y receptor activation.
Studies also indicate that cytochrome P450 metabolites, such as
20-hydroxyeicosatetraenoic acid (20-HETE), may play an important
role as second messengers facilitating P2 receptor activation in
renal microvascular (Renal microcirculation,kidney microcirculation)smooth muscle. 20-HETE is an important
modulator of L-type Ca2+ channel function, K+ channel function
and renal vascular autoregulatory responses [77¨C80]. In one
study, afferent arteriolar responses to ATP, ¦Á ¦Â-methylene ATP
and UTP were determined before and after treatment with the
selective CYP450 hydroxylase inhibitor,
N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS), or the
20-HETE antagonist, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid
(20-HEDE) [81]. The sustained vasoconstriction normally observed
in response to ¦Á ¦Â-methylene ATP under control conditions was
eliminated during 20-HETE inhibition with DDMS or 20-HEDE [81].
In contrast, afferent arteriolar vasoconstriction induced by
P2Y2 receptor activation with UTP was unaffected by inhibition
of 20-HETE [81]. The ATP-induced increase in intracellular
calcium concentration in preglomerular microvascular smooth
muscle cells was significantly attenuated by 20-HEDE [82].
Similarly, 20-HETE inhibition attenuated the increase in
intracellular calcium concentration induced by ¦Á ¦Â-methylene
ATP, but responses evoked by UTP were unchanged [82]. These
results demonstrate that 20-HETE plays a significant role in the
renal (Renal microcirculation,kidney microcirculation)vascular vasoconstrictor response and the elevation of
intracellular calcium concentration in response to P2X1 receptor
activation.
Rho-kinase and its multiple co-factors modulate vascular tone,
purportedly by increasing calcium sensitivity and thus smooth
muscle contractility [83¨C85]. The Rho-kinase system also
influences P2 receptor-mediated vasoconstriction,
20-HETE-dependent intracellular signalling, renal microvascular
(Renal microcirculation,kidney microcirculation)function and myogenic autoregulatory behaviour [86¨C93].
Inhibition of Rho-kinase in hydronephrotic kidneys vasodilates
virtually every pre- and postglomerular artery/arteriole and
attenuates endothelin- or adenosine-induced renal microvascular
vasoconstriction [87]. Similarly, preliminary in vitro studies
revealed that Rho-kinase inhibition with Y-27632 produced a
rapid, concentration-dependent afferent arteriolar vasodilation
and inhibited renal(Renal microcirculation,kidney microcirculation) autoregulatory responses [88]. In addition,
vasoconstrictor responses induced by angiotensin II or ATP were
markedly attenuated [88]. Interestingly, the afferent arteriolar
vasoconstriction elicited by ¦Á ¦Â-methylene ATP was completely
eliminated [88]. Thus, P2 receptor activation involves induction
of a number of second messenger systems that also play major
roles in regulating pressure-dependent renal perfusion.
P2 receptors and their roles in renal
autoregulation and tubuloglomerular feedback
The phenomenon of autoregulation is a critical renal
microvascular (Renal microcirculation,kidney microcirculation)control mechanism that serves to assure and
protect normal renal function [55, 94¨C98]. The autoregulatory
mechanism maintains a stable renal (Renal microcirculation,kidney microcirculation)blood flow (Fig. 3a), glomerular capillary pressure and glomerular filtration rate by
buffering acute changes in renal perfusion pressure with precise
adjustments in preglomerular vascular resistance [36, 55,
94¨C98]. Whole-kidney autoregulation integrates the combined
influences of two major regulatory systems. They include an
intrinsic myogenic mechanism operating along the preglomerular
vascular tree, and the tubuloglomerular feedback mechanism,
which regulates tone in the distal afferent arteriole through an
interaction between the arteriole and the macula densa region in
the thick ascending limb of the loop of Henle of the same
nephron [55, 95, 97¨C101]. The signalling mechanism(s) by which
changes in transmural pressure and/or stimulation of
tubuloglomerular feedback produce precise adjustments in
afferent arteriolar resistance remain somewhat controversial and
are under intensive study. This section of the review will try
to highlight the important controversies in the area but will
not go into exhaustive detail. The interested reader is referred
to some excellent reviews, which devote more attention to the
subject [8, 12, 28, 95, 97, 98, 102¨C106] (plus, see article by
Bell et al [107] in this Special Issue). Instead, this review
will focus on the critical data leading to the postulate that
ATP transduces haemodynamic and tubular information into
autoregulatory adjustments in afferent arteriolar diameter by
stimulating P2 receptors.
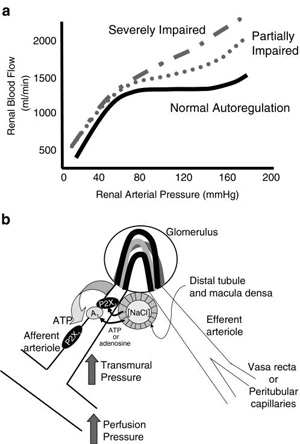 Fig.
3 Fig.
3
Postulated signalling mechanisms for ATP-mediated autoregulatory
adjustments in afferent arteriolar diameter. Panel a illustrates
a normal profile for autoregulation of renal (Renal microcirculation,kidney microcirculation)blood flow (solid
black line) and the autoregulatory profiles that might be ...
It is generally agreed that autoregulation is mediated by a
locally generated paracrine messenger molecule, or molecules,
linking myogenic and tubuloglomerular feedback signals from the
macula densa with highly precise adjustments in afferent
arteriolar resistance to modulate glomerular capillary pressure
and glomerular filtration rate [36, 55, 95, 97, 98]. Recently,
most attention has focused on the respective roles of adenosine
versus its precursor, ATP, as direct effectors of autoregulatory
vascular responses [33, 55, 95, 97, 98, 101]. The evidence for
adenosine in modulating tubuloglomerular feedback arises mainly
from micropuncture studies in rat kidneys. Pharmacological
blockade of A1 receptors blunts tubuloglomerular
feedback-dependent changes in proximal tubule stop-flow pressure
stimulated by increasing distal tubular NaCl concentration
[108¨C110]. Tubuloglomerular feedback responses are also
inhibited by suppression of adenosine formation from ATP using
an antagonist of ecto-5ˇänucleotidase (the enzyme that catalyses
the final reaction in the formation of adenosine), either alone
or in combination with continuous administration of an A1
receptor agonist (cyclohexyladenosine) to ˇ°clampˇ± endogenous
adenosine levels [109, 111]. Furthermore, mice deficient in
ecto-5ˇä-nucleotidase have attenuated tubuloglomerular feedback
responses [111, 112]. These observations support a primary role
for A1 receptors in mediating tubuloglomerular feedback
responses (Fig. 3b). Alternatively, the activation of
vasodilatory A2 receptors on afferent arterioles could blunt
autoregulatory and tubuloglomerular feedback responses under
conditions where A1 receptors are genetically
deleted/inactivated or they are blocked pharmacologically [54,
102, 113]. Nevertheless, renal (Renal microcirculation,kidney microcirculation)blood flow autoregulation is
inhibited by approximately 40% in adenosine A1
receptor-deficient mice and tubuloglomerular feedback responses
are attenuated [114, 115]. Interestingly, glomerular filtration
rate is normal in these mice. Collectively, these data support
the hypothesis that adenosine is an important paracrine
signalling molecule for transmitting tubuloglomerular feedback
and contribute to whole-kidney autoregulatory responses
[114¨C118].
However, other results suggest that adenosine and A1 receptors
are less important for overall autoregulatory resistance
adjustments in both in vivo and in vitro settings [30, 54, 113,
119]. Only modest inhibition of tubuloglomerular feedback
responses was observed during peritubular perfusion with
adenosine receptor antagonists or saturating concentrations of
adenosine [54], and autoregulation of renal (Renal microcirculation,kidney microcirculation)blood flow and glomerular filtration rate remains normal in the canine kidney
during adenosine receptor blockade [30, 119]. Micropuncture
studies revealed that blockade of adenosine A1 receptors dilated
rat afferent arterioles and their upstream arterial segments,
but tubuloglomerular feedback responses remained intact [34,
54]. Pressure-mediated vasoconstriction of rat juxtamedullary
afferent arterioles is unaffected by blockade of A1 receptors
[33, 120]. During saturation of adenosine receptors with high
doses of adenosine, marked vasodilation and loss of afferent
autoregulatory responses occurred via an A2 receptor mechanism
[113]. Blockade of A2 receptors alone or combined A2 and A1
receptor antagonism restored afferent arteriolar autoregulatory
capability [113]. Collectively, these studies suggest that
adenosine modulates autoregulatory responses via selective
activation of A1 and A2 receptors, but is not essential for the
manifestation of autoregulatory responses.
An alternative hypothesis is that extracellular ATP serves as
the primary signalling molecule mediating renal (Renal microcirculation,kidney microcirculation)autoregulatory
and tubuloglomerular feedback responses (Fig. 3b) [33, 43, 51,
54, 95, 97, 120¨C125]. Microdialysis of the renal cortical
interstitium reveals that the concentration of ATP in the
cortical interstitial fluid increases as renal arterial pressure
increases and as autoregulatory adjustments in renal vascular
resistance occur. Interstitial adenosine concentrations do not
change during this period [36, 120, 124, 125]. Renal (Renal microcirculation,kidney microcirculation)cortical
interstitial ATP concentration also increases when tubuloglomerular feedback responses are induced by increasing
distal volume delivery by means of acetazolamide treatment [120,
125]. Conversely, interstitial ATP concentration decreases when
tubuloglomerular feedback responses are inhibited with
furosemide [120, 125]. These manipulations of tubuloglomerular
feedback signals do not alter renal (Renal microcirculation,kidney microcirculation)interstitial adenosine
concentrations. In addition, pressure- and tubular flow-mediated
increases in renal cortical interstitial ATP concentration were
maintained in kidneys treated with the L-type Ca2+ channel
blocker, nifedipine, which completely prevented autoregulatory
adjustments in renal (Renal microcirculation,kidney microcirculation)vascular resistance, indicating that
changes in interstitial ATP concentrations precede changes in
renal vascular resistance [124]. Thus, interstitial ATP
concentrations are directly correlated with conditions that
evoke tubuloglomerular feedback and myogenic autoregulatory
responses, consistent with extracellular ATP mediating
autoregulatory adjustments in preglomerular vascular resistance
(Fig. 3b).
As indicated above, afferent arterioles represent the primary
preglomerular renal microvascular (Renal microcirculation,kidney microcirculation)element that sets and
regulates renal vascular resistance. Traditionally, direct
assessment of afferent arteriolar responses to vasoactive
stimuli has been accomplished either using isolated, cannulated
arterioles held on perfusion pipettes in vitro or using
hydronephrotic kidneys in vitro or in vivo. The in vitro blood-perfused
juxtamedullary nephron preparation presents a unique opportunity
to directly observe renal microvascular (Renal microcirculation,kidney microcirculation)function without
separating the vascular element from upstream or downstream
vascular segments and while maintaining the association of the
blood vessel with the tubular structures it serves [126¨C129].
Accordingly, this preparation provides a useful tool for
observing myogenic and tubuloglomerular feedback contributions
to renal (Renal microcirculation,kidney microcirculation)autoregulation [99, 130]. With this preparation,
pressure-mediated afferent arteriolar vasoconstriction is
markedly attenuated by P2 purinoceptor desensitisation, by
pharmacological blockade of P2 receptors or by genetic deletion
of P2X receptors [12, 33, 51, 131]. As shown in Fig. 4,
pressure-mediated afferent arteriolar autoregulatory
vasoconstriction was inhibited by non-selective P2 receptor
blockade with suramin or PPADS or by more selective P2X receptor
blockade with NF-279 [33, 51]. Furthermore, mice lacking P2X1
receptors exhibit impaired pressure-mediated afferent arteriolar
vasoconstriction [33]. While it was clear that deletion or
inactivation of P2X1 receptors inhibited autoregulatory
behaviour, it was not clear if this inhibition reflected loss of
myogenic behaviour or tubuloglomerular feedback influences. In
order to try to identify which component of autoregulation was
influenced by P2X1 receptor inactivation, experiments were
performed to delete the tubuloglomerular feedback component. The
rationale was that if tubuloglomerular feedback responses were
already absent in the P2X1 knockout mice, then interventions
designed to inhibit tubuloglomerular feedback responses would
have no effect on the autoregulatory response in these mice,
whereas the same interventions would attenuate overall
autoregulatory responses in normal wild-type mice by subtracting
the tubuloglomerular feedback contribution from the overall
autoregulatory response. Accordingly, tubuloglomerular feedback
responses were inhibited by either by resection of the loops of
Henle (papillectomy) or by exogenous administration of
furosemide. Acute papillectomy interrupts the flow of distal
tubular fluid past the macula densa and minimises
tubuloglomerular feedback-dependent influences on afferent
arteriolar function [33, 99, 132¨C134], while furosemide
administration has been used to inhibit tubuloglomerular
feedback responses by inhibiting the NKCC-2 transporter in the
apical membrane of the macula densa cells [33, 125, 132, 133,
135¨C141]. Using these strategies, it was clear that
pressure-mediated autoregulatory responses were significantly
blunted in wild-type mice, whereas furosemide or papillectomy
had no effect on the autoregulatory response observed in P2X1
receptor knockout mice [33]. This observation suggests that the
tubuloglomerular feedback component of the autoregulatory
response is already absent in mice lacking P2X1 receptors.
Further support for the P2X1 receptor hypothesis arises from the
observation that afferent arterioles from P2X1 knockout mice
vasoconstrict during A1 receptor stimulation with the A1
receptor agonist, N6-cyclopentyl adenosine, and A1
receptor-mediated vasoconstriction of rat afferent arterioles
was not affected by P2X1 receptor blockade with NF-279 [33].
Thus, P2X1 receptor knockout mice exhibit blunted autoregulatory
behaviour despite retaining a functional adenosine receptor
system. These data suggest that ATP-sensitive P2X1 receptors are
essential signalling components of pressure-mediated
autoregulatory behaviour and for translating macula densa
signals into tubuloglomerular feedback-mediated vasoconstriction
of afferent arterioles.
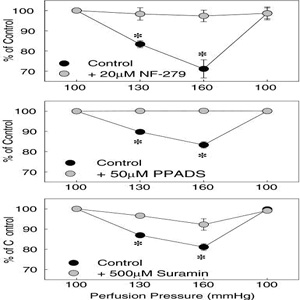 Fig.
4 Fig.
4
Effect of P2 receptor blockade on the afferent arteriolar
autoregulatory response induced by an increase in perfusion
pressure. Pressure-mediated afferent arteriolar autoregulatory
responses are depicted before and during P2 receptor blockade
with NF-279 ...
That ATP is a mediator of tubuloglomerular feedback signals is
supported by recent studies showing that macula densa cells
release ATP in response to conditions known to evoke
tubuloglomerular feedback responses [121¨C123]. Using a
microdissected glomerular preparation with an attached macula
densa, Bell et al [121] determined that ATP was released from
the basolateral aspect of the macula densa in response to a
tubuloglomerular feedback stimulus. They used biosensor cells
overexpressing P2X receptors to monitor cellular responses by
whole-cell patch clamp. When the biosensor was placed in close
proximity to the basolateral surface of the macula densa, a
tubuloglomerular feedback stimulus stimulated an increase in the
intracellular calcium concentration in the biosensor cell. The
calcium concentration in the biosensor cell also increased when
exposed to ATP directly, or when the NaCl concentration in the
distal tubular fluid was increased. The calcium response was
prevented if the biosensor was moved away from the macula densa.
These data argue that ATP, or another purinergic substance, is
released from the basolateral aspect of the macula densa into
the surrounding fluid.
Studies using the isolated perfused rabbit juxtaglomerular
apparatus, combined with confocal fluorescence imaging,
demonstrate propagation of a calcium signal from the macula
densa toward the proximal afferent arteriole, the adjacent
glomerulus and intraglomerular cells, in response to increasing
tubular flow past the macula densa [142]. Propagation of the
calcium signal and afferent arteriolar vasoconstriction are
inhibited by P2 receptor blockade but not by P1 receptor
blockade. Accordingly, ATP appears to represent a key signalling
molecule linking the macula densa with tubuloglomerular
feedback-mediated afferent arteriolar vasoconstriction [143].
Interestingly, increases in tubular fluid flow rate in isolated
perfused mouse thick ascending loops of Henle result in P2
receptor-mediated increases in epithelial cell cytosolic calcium
concentration [144]. This flow-induced elevation of
intracellular calcium concentration was almost completely
blocked when the non-selective P2 receptor antagonist, suramin,
or the ATP scavenger, apyrase, was added to the bath solution.
These data suggest that increasing tubular fluid flow and/or
tubular distention stimulate ATP release from the basolateral
aspect of the renal (Renal microcirculation,kidney microcirculation)tubule into the adjacent bathing medium.
Addition of suramin or apyrase to the luminal perfusate also
significantly blunted the perfusion-induced increase in
intracellular calcium concentration in the tubular epithelial
cells. The change in fluid flow is postulated to be detected by
mechanical stimulation of a primary cilium extending into the
tubular lumen from the apical membrane of the epithelium. This
study suggests that mouse thick ascending loop of Henle tubular
epithelial cells are capable of detecting changes in tubular
distention and/or tubular fluid flow rate and respond by
releasing ATP into the tubular fluid and into the ˇ°interstitial
fluidˇ± adjacent to the basolateral surface of the tubule.
Interestingly, similar observations have also been made for
other renal (Renal microcirculation,kidney microcirculation)tubular epithelia. Mechanical stimulation of apical monocilia has been found to regulate ATP release from cultured
mouse collecting duct principal cells [145]. Flow-induced ATP
release was robust in cilium-competent monolayers of epithelial
cells but significantly blunted in cilium-deficient monolayers
[145]. This may represent an important sensory system used by
macula densa cells to monitor tubular fluid flow and thereby
influence macula densa ATP release. Macula densa cells extend a
single cilium from the apical membrane into the tubular lumen
[146]. Thus, to extrapolate from observations made in other
renal (Renal microcirculation,kidney microcirculation)epithelia to the macula densa, mechanical stimulation of
the macula densa cilia may trigger ATP release from the
basolateral membrane into the interstitial fluid adjacent to the
basolateral surface of the macula densa plaque and initiate
tubuloglomerular signals to the afferent arteriole.
Metabolism of extracellular ATP involves ectonucleotidases that
are highly expressed in the kidney [7, 147¨C153] (plus see
article by Shirley, Vekaria & S¨¦vigny [154] in this Special
Issue). Ectonucleotidases cleave ATP to AMP and 5ˇä-nucleotidase
hydrolyzes AMP to adenosine. These ecto-enzymes may represent a
mechanism by which the interstitial adenosine concentration is
regulated through systematic degradation of ATP in the renal
(Renal microcirculation,kidney microcirculation)interstitial fluid. Thus, ectonucleotidases may catabolize ATP
released from the macula densa to yield adenosine that can
activate P1 receptors. To distinguish P2 receptor-mediated
effects from P1 receptor effects, various pharmacological,
biochemical and molecular tools have been used, including
metabolically stable P2 receptor agonists, P1 and P2 receptor
antagonists, adenosine uptake inhibitors and ectonucleotidase-deficient
mice. In isolated perfused rat kidneys, the vascular response to
ATP is unaltered by the adenosine receptor antagonist,
8-phenyltheophylline or the adenosine uptake inhibitor, S-(p-nitrobenzyl)-6-thioinosine
[45]; indicating that P2 receptors are directly activated by
ATP. In juxtamedullary nephrons, ATP-mediated vasoconstriction
of afferent arterioles is enhanced during adenosine receptor
blockade [21]. However, Takenaka et al reported that enhanced
production of adenosine from AMP by intrarenal infusion of 5ˇä-nucleotidase
improved autoregulation in Thy-1 nephritic rats, which are a
model of mesangial cell ablation pathology associated with
impaired autoregulation [153]. Moreover, tubuloglomerular
feedback stop-flow pressure responses are attenuated by
inhibition of adenosine formation with the 5ˇä-nucleotidase
blocker, ¦Á ¦Â-methylene ADP [109], and by deletion of the gene
coding for ecto-5ˇä-nucleotidase (CD73) expression [111, 112].
However, if ATP is not metabolised and its concentration
increases in the interstitial fluid, it is possible that it
could desensitize P2 receptors and thereby lead to reduced
tubuloglomerular feedback reactivity.
Studies were recently performed to assess the ability of the
cells making up the glomeruli to catabolize ATP to its main
degradation products: ADP, AMP and adenosine [151]. Incubation
of isolated glomeruli under control conditions revealed that
basal extracellular ATP concentrations in the bathing medium
reached 1.0 nM and increased approximately 6.5-fold when
ecto-ATPase activity was inhibited with 6-N,
N-diethyl-¦Â-¦Ă-dibromomethylene-d-adenosine-5-triphosphate
(ARL67156) [151]. In the absence of ecto-ATPase inhibitor,
exogenous ATP added to these isolated glomeruli was almost
completely hydrolyzed within 20 min, with AMP being the major
catabolic product. AMP has little effect on afferent arteriole
diameter [151]. Mechanical perturbation of isolated glomeruli
during inhibition of ecto-ATPase activity increases the ATP
concentration in the bathing medium by approximately 35%. These
data demonstrate that ATP is constitutively released from
isolated rat glomeruli and that this release is enhanced by
mechanical stimulation or glomerular deformation. Glomeruli
express detectable ecto-5ˇä-nucleotidase activity which can be
modulated by dietary salt [152].
A recent report suggests a potential interaction between ATP-
and adenosine-mediated autoregulatory influences and connexins
37 and 40 [155]. Blockade of connexins 37 and 40 attenuated
autoregulatory behaviour by approximately 50%. Blunting of the
autoregulatory response with connexin blockers was augmented by
A1 receptor blockade but not by P2 receptor blockade. However,
when A1 and P2 receptor blockers were used together, the impact
of connexin inhibitors was significantly enhanced. These data
suggest that A1 receptors and P2 receptors may interact with
each other and with connexins 37 and 40 to transduce purinergic
autoregulatory signals intended to regulate preglomerular
resistance. The authors concluded that A1 and P2 receptors are
needed to achieve complete whole-kidney autoregulatory behaviour
and suggested that A1 and P2 receptors interact in that
response.
Purinoceptors in hypertension
Hypertension is frequently associated with progressive renal
(Renal microcirculation,kidney microcirculation)injury that develops through a number of poorly understood
factors [156, 157]. Compromised renal autoregulatory efficiency
can be a contributing factor that results in chronic elevation
of glomerular capillary pressure and subsequent glomerular
injury [96]. Renal(Renal microcirculation,kidney microcirculation) autoregulation is impaired in many
experimental models of hypertension [106, 158¨C165]. Given that
purinoceptors are important for regulation of renal
microvascular function and autoregulation, and for modulation of
haemodynamic function and tubular transport, it is reasonable to
examine the relationship between purinoceptor function and
hypertension-related renal injury. Studies indicate that
purinoceptors contribute to the functional adaptations in the
development of hypertension and may contribute to the renal
pathophysiology of hypertension [162, 166, 167]. P2X7 receptor
immunoreactivity is increased in mesangial cells from Ren-2
transgenic hypertensive rats [166]. Renal(Renal microcirculation,kidney microcirculation) injury in Ang-II-infused
hypertensive rats is ameliorated by treatment with the P2Y12
receptor antagonist, clopidogrel, as well as the non-selective
P2 receptor blocker, PPADS, without reducing arterial pressure
[168]. Pressure-mediated afferent arteriolar autoregulatory
responses are attenuated in Ang-II-infused hypertensive rats
[162, 169]. Consistent with impaired autoregulatory behaviour,
afferent arteriolar vasoconstrictor responses to ATP and ¦Â ¦Ă-methylene
ATP are markedly attenuated compared with those seen in
normotensive controls (Fig. 5a), supporting the hypothesis that
P2 receptors are essential elements in the autoregulatory
response. Interestingly, afferent arteriolar responses to the P1
receptor agonist, adenosine, were unchanged [11]. P2X1
receptor-mediated impairment of afferent arteriolar
vasoconstriction is associated with impaired calcium signalling
responses to ATP or ¦Â ¦Ă-methylene ATP in freshly isolated
preglomerular smooth muscle cells from hypertensive rats
compared to normotensive controls (Fig. 5b) [11]. Reduction of
the calcium signalling response could account for the impaired
afferent arteriolar autoregulatory efficiency and thus
contribute to hypertension-induced renal (Renal microcirculation,kidney microcirculation)and glomerular injury.
Attenuated responsiveness to P2 receptor stimulation in this
model of hypertension is counter to responses observed with
other vasoconstrictor agonists. For example, afferent arteriolar
responses to angiotensin II are enhanced in Ang-II-infused
hypertensive rats whereas the response to noradrenaline and
adenosine are unchanged [160, 170]. Simultaneous loss of
autoregulatory responsiveness and responsiveness to P2X1
receptor activation is interesting and supports the argument
that normally functioning P2X1 receptors are essential for
normal autoregulatory behaviour to occur.
Perspectives
P2 receptors in the control of renal (Renal microcirculation,kidney microcirculation)tubular and haemodynamic
function remain an emerging and exciting field of study.
Evidence that P2 receptors are ubiquitously expressed throughout
the kidney but that the receptor expression profile is different
between different nephron segments and structures argues that
each serves an important physiological function. Studies have
not progressed far enough to definitively assign function to
receptors expressed in each kidney region. However, compelling
data are accumulating that implicate P2 receptors as
contributing importantly to both physiological and
pathophysiological processes. There is much to be learned about
this class of receptors and there is much excitement ahead as
their respective roles are defined.
It is clear that P1 and P2 receptors are effective modulators of
renal microvascular (Renal microcirculation,kidney microcirculation)function. P2X1 receptors are important for
pressure-dependent autoregulatory adjustments in renal vascular
resistance. The debate continues on whether or not they are
important for tubuloglomerular feedback adjustments in afferent
arteriole resistance. Many other questions remain to be
answered. What is(are) the actual messenger molecule(s)
responsible for autoregulatory changes in renal(Renal microcirculation,kidney microcirculation) vascular
resistance? Is it ATP, adenosine or another yet unidentified
substance? Are the myogenic and the tubuloglomerular feedback
components of the whole-kidney autoregulatory response mediated
by the same messenger molecule? What is the role of renal
ectonucleotidases in the physiology of the purine signalling
pathways described in this review? Is autoregulatory impairment
directly responsible for hypertension-related kidney injury or
are there other more important intermediates involved? If
failure of the renal(Renal microcirculation,kidney microcirculation) vascular purinoceptor system is responsible
for some aspects of hypertensive renal injury, could it also be
involved in renal injury in other conditions such as diabetes,
obesity or immunosuppressive therapies? Answers to these and
other questions will help push development of this field and
will contribute greatly to our understanding of why these
tissue-specific renal (Renal microcirculation,kidney microcirculation)purinoceptor receptor systems are
distributed the way they are. Time and better investigational
tools will help to clarify the physiology of these receptors.
Nevertheless, the data generated to date clearly establish the
renal (Renal microcirculation,kidney microcirculation)P2 receptor system as an important regulatory system for
maintaining renal vascular and tubular function.
Acknowledgements
The author wishes to thank Dr. Zhengrong Guan for her assistance
in preparing this manuscript. Some of the work described in this
review was supported by grants from the American Heart
Association and by NIH (DK44628 and HL 074167).
References
1. Bailey MA, Hillman KA, Unwin RJ (2000) P2 receptors in the
kidney. J Auton Nerv Syst 81:264¨C270. [PubMed]
2. Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K,
Burnstock G, Unwin RJ (2000) Axial distribution and
characterization of basolateral P2Y receptors along the rat
renal tubule. Kidney Int 58:1893¨C1901. [PubMed]
3. Bailey MA, Turner CM, Hus-Citharel A, Marchetti J,
Imbert-Teboul M, Milner P, Burnstock G, Unwin R (2004) P2Y
receptors present in the native and isolated rat glomerulus.
Nephron Physiol 96:79¨C90 .
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2776135/ |