|
Introduction The microcirculation(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument) is the
part of the vascular system that consists of arterioles,
capillaries and venules. It plays a pivotal role in tissue
oxygen delivery (1). Microcirculatory dysfunction can be
observed in critically ill patients - e.g. in sepsis and during
cardiac surgery- and is associated with an adverse prognosis
(2-5). Recent research has focused on both the investigation of
microcirculatory dysfunction as well as the effect of
interventions on microcirculatory alterations. The
microcirculation can be visualized with Sidestream Dark Field (SDF)
imaging (see below for further description). Several single
center studies have demonstrated an association between
microcirculatory (sidestream dark field (SDF)
handheld imaging device,Microvascular
(blood) image observation instrument) dysfunction and severity of illness, but an
international observational study has not been conducted this
far.
SDF imaging
Orthogonal Polarizing Spectral (OPS) imaging (sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument)was initially introduced to
the clinic for observation of the microcirculation at the
bedside by the Department of Physiology at the Academic Medical
Center of the University of Amsterdam, the Netherlands. The
technique consists of a handheld microscope with a light guide
and a disposable sterile lens at the tip. This light guide with
a lens at its tip is placed on tissue (e.g. sublingual or
intestinal mucosa), and polarized light with a wave length
within the absorption spectrum of hemoglobin is emitted. Crossed
polarized detection of the images allows filtering out of the
surface reflection of the emitted light thus leaving the light
from the deeper layers where the microcirculation is to be
imaged. Since the emitted wavelength is absorbed selectively by
hemoglobin in the erythrocytes, the flowing cells can be imaged
as dark corpuscles flowing through the microcirculation(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument). In this
way the perfusion (e.g. flow) as well as the density of the
functional capillaries can be determined at the bed side (6).
Currently, Sidestream Dark Field (SDF) imaging is applied
(figure 1). This technique is essentially the same as OPS
imaging but uses side illumination of the area under
investigation instead of polarized light: the lens is optically
isolated from the outer ring with LEDs, thereby preventing the
influence of surface reflections. Therefore, SDF offers a better
image quality (7). The cited studies offer a detailed
description of both techniques.
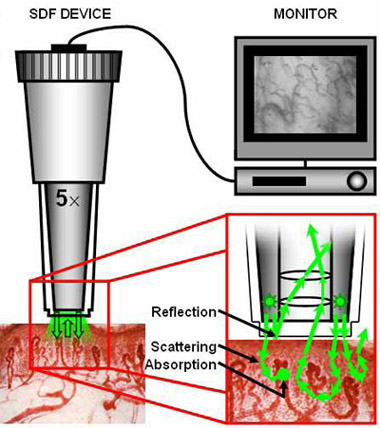
Figure 1. Left: The hand-held Sidestream Dark Field (SDF)
imaging device- equipped with a 5
magnifying objective lens system- imaging the tissue-embedded
microcirculation by the use of green pulsed LED ring
illumination. Upper right corner: Images are recorded using a
digital
video recorder/computer and visualized on a monitor. Lower right
corner: After penetration into the tissue, the illumination
light undergoes scattering events (indicated with arrows) and
can be absorbed
by (de)oxyhemoglobin (indicated with dots). The SDF(Sidestream
Dark Field(SDF),sidestream
dark field (SDF) imaging,Side
stream
dark field imaging (SDF),Sidestream
dark field imaging (SDF)) lens system
is optically isolated from the
illuminating outer LED ring so that there is no contamination of
the microcirculatory(sidestream dark
field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument) images by
surface reflections. Adapted from: (7).
No adverse effects on patient well being have been observed
during OPS and SDF studies(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument). Study design The aim of this
international multi-center observational study is to investigate
the prevalence of sublingual microcirculatory alterations in
intensive care unit (ICU) patients, regardless of their
underlying disease, monitored at a single moment in time in the
different centers. In patients with an intestinal stoma, SDF
images of intestinal mucosa will also be obtained. A similar
multi-central prevalence study had been carried out in ICU
patients concerning the severity of disease. In this well known
Sepsis Occurrence in Acutely ill Patients (SOAP) study clinical
measurements and patient characteristics were recorded at a
single time point in numerous intensive care units throughout
the world (8). It is our intention to use a similar study design
where we investigate the prevalence of microcirculatory
(sidestream dark field (SDF) handheld
imaging device,Microvascular (blood)
image observation instrument) alterations in intensive care patients and the relationship of
microcirculatory alteration to the severity of disease in an
epidemiological survey. A specifically designed website (www.microcirculationstudies.org)
using dedicated clinical trial software (Open Clinica 3.0,
www.openclinica.org), constructed in accordance with
international guidelines (e.g. 21 CFR Part 11 (FDA), ICH-GCP and
the Health Insurance Portability and Accountability Act of 1996
(HIPAA)) will be available for data exchange. This study is
registered at ClinicalTrials.gov (NCT01179243).
Patient selection Inclusion criteria: - ICU patients ¡Ý 18 years
- Informed consent Exclusion criteria - Recent maxillofacial
surgery
- Injury to the maxillofacial area (ulceration, mucosal
bleeding) - Participation in other clinical research is no
exclusion criterion, except when this is contradictory to local
legislation.
Methods To investigate the prevalence of microcirculatory
(sidestream dark field (SDF) handheld
imaging device,Microvascular (blood)
image observation instrument) dysfunction, SDF(Sidestream
Dark Field(SDF),sidestream
dark field (SDF) imaging,Side
stream dark field imaging (SDF),Sidestream
dark field imaging (SDF)) measurements of the sublingual microcirculation
(and intestinal microcirculation, if applicable) will be made in
several ICU¡¯s, at a time point stipulated by the steering
committee. In addition, information on patient characteristics
is collected.
Thirty ICU¡¯s worldwide, with access to SDF equipment, will be
invited or are already invited to participate. Because this is
the first extensive prevalence study on microcirculatory(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument)
alterations, with a primarily explorative character, making a
solid estimation of the study size is difficult. However, an
approximation of the study size can be calculated from the
aforementioned SOAP study, smaller studies on microcirculatory
dysfunction in, mainly, sepsis patients and advice from H. Groen,
PhD, statistician Medical Center Leeuwarden, the Netherlands
(appendix). Descriptive studies on microcirculatory (sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument) dysfunction
in sepsis have included 26-50 patients (2,4,9). In a
comparatively small group of 25 patients after major abdominal
surgery, the researchers were able to detect an association
between microcirculatory changes and the development of
complications (5). 37% out of the total SOAP study population of
3147 patients were diagnosed with sepsis (8). Supposing that the
approximate prevalence of sublingual microcirculatory (sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument)
alterations in sepsis is 75% and choosing a precision of 5%, our
goal is to include 284 patients with sepsis (see table
¡®Confidence interval of a
proportion, which is added as a supplement). Supposing that
these sepsis patients constitute 37% of the ICU-population, we
should aim for 768 patients as a total study size. However,
inclusion may be limited due to availability of SDF equipment
and occupation of ICU beds. We expect inclusion of around 100
patients of Dutch ICU¡¯s.
The SDF/OPS measurements will be performed by employees of the
participating ICU¡¯s. Analysis will be performed at random,
blinded and anonymous (i.e. to prevent recognition of patients
and participating centers), according to previously published
guidelines (10,11). Researchers, selected by the steering
committee, of the Dept. of Intensive Care, Medisch Centrum
Leeuwarden (Dr E.C. Boerma) and at the Dept. of Translational
Physiology (Prof Dr C. Ince) Academic Medical Center, University
of Amsterdam, the Netherlands, will take care of the analysis.
The microcirculation can be described in terms of both flow (microvascular
flow index (MFI), ranging from 0 = no flow to 3 = continuous
flow) and diffusion (proportion of perfused vessels (PPV) and
functional capillary density (FCD)). The cited articles (10,11)
give a detailed description. We expect not only to obtain
important information concerning the clinical significance of
microcirculatory alterations, but we also expect that this study
will provide the basis for conducting interventional studies in
the future, targeting improvement of the microcirculation(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument). Dutch
legislation allows anonymous medical data to be filed for over
15 years (12).
After analysis of the SDF-images(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument), further statistical analysis
will be conducted to relate the microcirculatory alterations to
the severity of disease and other parameters. The primary
outcome measure is the association between microcirculatory
changes and different disorders, secondary outcome measures are
associations between microcirculatory(sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument) changes and length of
ICU/hospital stay, illness severity, mortality and several haemodynamic parameters such as cardiac index and blood
pressure. The other collected variables serve to describe the
study population. Normally distributed variables (as tested by
for instance the Kolmogorov-Smirnov test) will be displayed as
mean/standard deviation, non-normally distributed variables as
median/interquartile range. Differences between several groups
will be assessed using a t-test in case of normally distributed
variables; in case of non-normally distributed variables a
non-parametric test will be chosen. Whenever possible,
regression analysis will be used to test for associations
between the severity of microcirculatory (sidestream
dark field (SDF) handheld imaging device,Microvascular
(blood) image observation instrument) dysfunction and illness
severity, mortality and length of stay.
Per patient, SDF images (3 steady video clips of 10-20 seconds
each)(Sidestream
Dark Field(SDF),sidestream
dark field (SDF) imaging,Side
stream dark field imaging (SDF),Sidestream
dark field imaging (SDF)) will be obtained. A template will be provided by the
steering committee for the following data to be obtained per
patient :
Demographic
variables
o
Age, sex
o
Length, weight
o
Diagnosis/reason
of ICU admittance
o Medical history:
e.g. diabetes, vascular disease
o
APACHE II,
cumulative SOFA
(13,14)
o
Time since ICU
admittance (afterwards: total length of ICU/hospital stay)
o ICU and hospital
mortality
Laboratory
variables on day of SDF imaging (routine measurements)(Sidestream
Dark Field(SDF),sidestream
dark field (SDF) imaging,Side
stream dark field imaging (SDF),Sidestream
dark field imaging (SDF)):
hemoglobin (Hb), hematocrit (Ht), arterial blood gas, arterial
lactate, C-reactive protein, leukocytes, thrombocytes,
prothrombin time (PT), activated partial thromboplastin time (APTT),
lactate |