|
Little is known about any changes in cerebral
hemodynamics(Cerebral microcirculation,
brain microcirculation),during and after abdominal aortic crossclamping and
unclamping, especially in the cerebral microcirculation(brain microcirculation). We
studied the effects of abdominal aortic cross-clamping and
unclamping on cerebral pial vessel diameter in the presence or
absence of the thromboxane (Tx)A2 receptor antagonist using a
closed cranial window in 27 rabbits. Although infrarenal aortic
cross-clamping did not affect pial vessel diameter, release of a
20-min aortic cross-clamp caused pial arterioles to dilate and
then constrict.Asignificant constriction persisted for at least
60 min (maximum, 17% for large [ 75 m] and 28% for small
arterioles [ 75 m] compared with baseline). Topical
administration of a TxA2 receptor antagonist, seratrodast, at 10
7 M and 10 6 M, significantly attenuated the constriction of
large and small arterioles (at 60 min, 9% and 13% constriction
for 10 7 M, and 6% and 7% for 10 6 M). Release of a 20-min
aortic cross-clamp induced a sustained pial arteriolar
constriction. Because this unclamping-induced vasoconstriction
was attenuated by topical administration of seratrodast, it was
likely partially mediated via the washout of TxA2 produced in
the ischemic region during the clamp and after crossclamp
release.
A brupt changes in systemic hemodynamics occur during and after
abdominal cross-clamping and unclamping performed to facilitate
abdominal aortic aneurysmectomy. Abrupt hypertension can occur
after cross-clamping, and severe hypotension after
unclamping. Although such hemodynamic instability would be
expected to affect the cerebral(Cerebral microcirculation,
brain microcirculation) circulation,only a few studies
have addressed this important question in humans (1). Liu et al.
(1) partially characterized the response of the
cerebral microcirculation(brain microcirculation) during and after aortic clamping and noted that
middle cerebral (Cerebral microcirculation,
brain microcirculation)artery blood flow velocity (VMCA) decreased
after infrarenal aortic cross-clamping in anesthetized patients,
and increased after unclamping.
In addition to the possible abrupt hemodynamic changes during
and after aortic clamping, many factors might affect the
cerebral microcirculation(brain microcirculation). These include accumulation of carbon
dioxide (CO2), desaturation of venous blood with a decreased pH
(2), and release into the general circulation of substances that
had accumulated in the peripheral vasculature below the aortic
clamp (such as K , prostaglandins [PGs], cytokines, endothelins,
anaphylatoxin, and neutrophils) (3¨C7). Kretzschmar et al. (8)
reported that the plasma levels of thromboxane (Tx)B2 (a
metabolite of TxA2) and 6-keto-PGF1 (a stable hydrolysis product
of PGI2) increased significantly after unclamping in
anesthetized patients undergoing abdominal aortic aneurysmectomy.
TxA2, a potent vasoconstrictor, may be responsible for the
development of pulmonary hypertension after unclamping (6).
Aadahl et al. (9) observed,in pigs, that cerebral (Cerebral microcirculation,
brain microcirculation)flux decreased
after unclamping of the thoracic aorta using laser Doppler
technique. In the present study, using the intracranial window
technique in rabbits, we examined the changes in cerebral(Cerebral microcirculation,
brain microcirculation) pial
arteriolar diameter during and after aortic clamping and
unclamping. In addition,we examined whether seratrodast, a TxA2
receptor antagonist, would affect these responses, and also
evaluated the plasma TxB2 level after unclamping a 20-min aortic
clamp.
Materials and Methods
The procedures used in the present study
conformed to the Guiding Principles in the Care and Use of
Animals approved by the Council of the American Physiologic
Society, and the experimental protocols were approved by our
Institutional Committee for Animal Care. The experiments were
performed on 27 anesthetized rabbits weighing 2.0¨C2.2 kg. Each
animal was initially anesthetized with pentobarbital sodium(25
mg/kg body weight, IV), and then anesthesia maintained with a
continuous infusion of the same drug (5 mg ˇ¤ kg 1 ˇ¤ h 1).
Mechanical ventilation was provided via a tracheotomy tube using
oxygenenriched room air (arterial O2 content, 14¨C17 vol%). The
tidal volume and respiratory rate were continually adjusted to
maintain Petco2 between 35 and 40 mm Hg; Petco2 was monitored
throughout the experiment. Polyvinyl chloride catheters were
placed in the femoral vein for the administration of fluid, in
the right axillary and left femoral arteries for the continuous
monitoring of proximal and distal aortic pressures (PrAP and
DiAP) and heart rate (HR), and also for blood sampling from the
right axillary artery. Rectal temperature was maintained between
38.5ˇă and 39.5ˇăC with a heating blanket and warming lamp. A skin
incision was made at the lateral abdomen. The aorta was then
taped in preparation for the clamp just distal to the renal
arteries.
A closed cranial window was used to observe the cerebral (Cerebral microcirculation,
brain microcirculation)pial
microcirculation(Cerebral microcirculation,
brain microcirculation). Each animal was placed in the sphinx posture,
the scalp was retracted, and a 10-mm-diameter hole was made in
the parietal bone.The dura and arachnoid membranes were opened
carefully, and a polypropylene ring with a glass coverslip was
placed over the hole and secured with dental acrylic. The space
under the window was filled with artificial cerebrospinal fluid
(aCSF). The composition of the aCSF was Na 151 mEq/L, K 4 mEq/L,Ca2
3 mEq/L, Mg2 1.3 mEq/L, Cl 110 mEq/L,HCO3 25 mEq/L, urea 40 mg/dL,
and glucose 67 mg/dL; pH was adjusted to 7.48. This solution was
freshly prepared each day, and bubbled with 5% CO2 in air at
39.0ˇăC for 15 min just before use. Four polyethylene catheters
were inserted through the ring; one was attached to a reservoir
bottle containing aCSF to maintain the desired level of
intrawindow pressure(5 mm Hg), the second was used to monitor
intrawindow pressure, the third for the administration of
experimental drugs and aCSF, and the fourth for draining the
fluid. Temperature within the window was monitored by using a
thermometer (model 6510;Mallinckrodt Medical, St. Louis, MO) and
was between 38.5ˇă and 39.5ˇăC. The volume below the window was
between 0.4 and 0.6 mL.
The diameters of two large ( 75 m) and two small( 75 m) pial
arterioles were measured in each cranial window by using a
videomicrometer on a television monitor attached to a
microscope(model SZH-10; Olympus, Tokyo, Japan). The data from
the pial views were stored on videotape for later playback and
analysis. The percent changes recorded for individual arterial
segments were averaged for each vessel type (large or small) in
each rabbit, and this average value was used in the statistical
analysis. These pial arteriolar diameters were within the range
40¨C120 m.Rabbits were assigned to one of three groups (see
below). All experiments were performed after at least 30 min
recovery from the surgical preparation. After baseline
measurements had been made, each rabbit was infused under the
window with one of the following:aCSF (control group, n 7), 10 7
M seratrodast in aCSF (seratrodast 10 7 M group, n 7), or 10
6Mseratrodast in aCSF (seratrodast 10 6Mgroup,n 7). All
infusions were at a rate of 0.25 mL/min throughout the
experiment. Each solution was freshly dissolved in aCSF for the
present study. Fifteen minutes after the start of topical
infusion, aortic clamping was performed for 20 min. The clamping
and unclamping were done gradually (each taking about 30 to
perform) so as to minimize hemodynamic changes.Measurements of
cerebral (Cerebral microcirculation,
brain microcirculation)pial arteriolar diameter, hemodynamic variables (PrAP,
DiAP, and HR), and physiologic variables (rectal temperature,
intrawindow temperature, arterial blood gas tensions,
electrolytes,blood sugar, and blood pH) were measured at the
following time points: just before the start of topical
administration (baseline), after 15 min topical administration
(Pre-Clamp), just after aortic clamping (After Clamp), 20 min
after clamping (Pre-Unclamp),and at 0, 2, 5, 15, 30, and 60 min
after unclamping. The time of 0 min after unclamping was 30 s
after the start of the unclamping.
In an additional experiment (n 6), the arterial concentration of
TxB2 (a stable metabolite of TxA2)was measured at baseline, 5,
and 60 min after unclamping during aCSF infusion under the
window (same as control group). The arterial blood was collected
from the right axillary artery. Plasma TxB2 was determined by
using a RIA kit (New England Nuclear,Boston, MA). The assay
sensitivity was 3.0 pg/mL. All variables used to assess the
time-dependent effects within groups and plasma TxB2 level were
tested by a one-way analysis of variance for repeated
measurements followed by the Scheffe´ F test for post hoc
comparisons. Differences between groups were examined by a
one-way analysis of variance for factorial measurements followed
by the Scheffe´ F test. Significance was considered to be
demonstrated at P 0.05.All results were expressed as mean sd.
Results
There were no significant differences in baseline hemodynamic or
physiologic variables among the groups. HR did not vary
significantly throughout the experiments in any group. In
addition, neither rectal temperatures nor intrawindow
temperatures were changed at any stage of the experiments in any
group. Pao2, Na , K , and blood sugar were stable at all stages
of the experiments in all groups. PrAP decreased significantly
at the point of 0 min after unclamping (P 0.05), and DiAP
decreased significantly after clamping in every group (P 0.05)
but then recovered after unclamping (Tables 1 and 2). Arterial
pH decreased significantly at 0 (P 0.05) and 2 min after
unclamping (P 0.05) in every group. Paco2 increased
significantly at 0 (P 0.05) and 2 min after unclamping (P 0.05)
in every group.There were no significant differences among the
groups in the baseline diameters of the large or small
arterioles In the control group, neither large nor small pial
arterioles showed significant changes in diameter after
clamping, but both types dilated significantly just after
unclamping (maximal increase: 6% and 10% above baseline,
respectively). They then constricted significantly starting at 5
min after unclamping ( 6% and 5% compared with baseline,
respectively). The constrictions were still significant (and
indeed appeared to be progressively constricting) at 60 min
after unclamping (Figs. 1 and 2).In the seratrodast 10 7 M and
10 6 M groups, baseline pial arteriolar diameters (large and
small) did not change as a result of topical seratrodast
administration or after clamping. However, large and small pial
arterioles dilated significantly just after unclamping; the
maximal increases in diameter were 7% and 9% for 10 7 M
seratrodast, and 7% and 12% for 10 6 M seratrodast (P 0.05).
These dilations were not significantly different from those seen
in the corresponding control group. However, the pial arteriolar
constriction observed 5 min after unclamping in the control
group was significantly attenuated by seratrodast in both large
and small arterioles (constriction at 5 min after unclamping, 2%
and 1% for 10 7 M seratrodast, 1% and 1% for 10 6 M seratrodast;
constriction at 60 min after unclamping, 9% and 13% for 10 7 M
seratrodast, 6% and 7% for 10 6 M seratrodast)(Figs. 1 and 2).
For each of the three groups,the small arterioles tended to be
more reactive than the large vessels (but not significantly so)
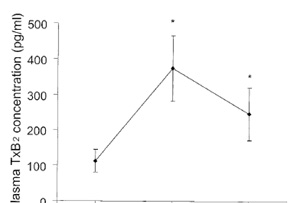
(Figs. 1 and 2). In the additional experiment, unclamping caused
the arterial TxB2 concentration to increase significantly from
104 17 (baseline) to 375 90 (5 min after unclamping) (P 0.05)
and 237 83 pg/mL (60 min after unclamping) (P 0.05) (Fig. 3).
Discussion
Our present findings indicated that the release of an aortic
clamp caused a transient dilation of cerebral(Cerebral microcirculation,
brain microcirculation) pial arterioles
for about 2 minutes, followed by a sustained vasoconstriction
for at least 60 minutes. To minimize abrupt hemodynamic changes
caused by abdominal aortic clamping and unclamping, and to avoid
potential effects of such changes on the cerebral microcirculation,we clamped and unclamped gradually (taking
about 30 seconds for each maneuver). In fact, except for the
point of zero minutes after unclamping,changes in PrAP were
insignificant in all groups throughout the experimental period
without any interventions.
The cerebral (Cerebral microcirculation,
brain microcirculation)vasoconstriction was significantly attenuated by
topical administration of seratrodast,a TxA2 receptor antagonist
suggesting dependence on activation of TxA2 receptors within the
central nervous system vasculature. We also showed that plasma
TxB2 (a metabolite of TxA2) concentration increased
significantly after unclamping, with a sustained increase at 60
minutes after unclamping.Tissue damage by ischemia leads to an
activation of the arachidonic acid cascade and consequent
generation of TxA2 and PGI2, and to activation of circulating
polymorphonuclear leukocytes (8,10). Reperfusion of organs and
tissues can induce a systemic reaction termed
ischemia-reperfusion syndrome, and certain substances (see
below), when washed out from the area of ischemia, could
conceivably cause damage to the microcirculation (Cerebral microcirculation,
brain microcirculation)in a remote
organ, such as the brain(Cerebral microcirculation,
brain microcirculation). Numerous vasoactive metabolites,
including TxA2 (8,10¨C12), lactate (10), renin (10,13),
angiotensin(10), endothelin-1 (14), epinephrine (10,15),
norepinephrine (10,15) and PGI2 (8,10), are formed in and washed
out from ischemic tissues distal to a clamp.
TxB2 and 6-keto-PGF1 have been reported to increase
significantly in the plasma after unclamping during abdominal
aortic aneurysmectomy under general anesthesia and the plasma
TxB2 level remained high until the end of surgery (8), findings
consistent with our observations. Taking those results together
with the present findings leads us to speculate that the
persistent cerebral(Cerebral microcirculation,
brain microcirculation) vasoconstriction seen after aortic
unclamping was mediated, at least in part, via a washout of TxA2
produced in distal tissues during the period the aorta was
clamped, and probably after cross-clamp release.
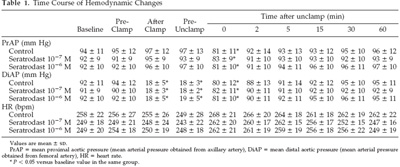
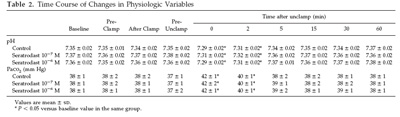
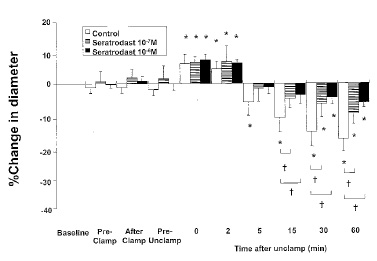
Figure 1. Effects of topical infusion of seratrodast on
reactivity of large cerebral (Cerebral microcirculation,
brain microcirculation)pial arterioles ( 75 m) to aortic
clamping and unclamping in 21 rabbits. Data are expressed as
percentage change from the diameter measured just before topical
administration of
drug (baseline). Data are shown for 15 min after topical
administration (Pre-Clamp), just after clamping (After Clamp),
20 min after
clamping (Pre-Unclamp), and 0, 2, 5, 15, 30, and 60 min after
unclamping. Values are mean sd. *P 0.05 compared with
baseline in the same group; †P 0.05 as indicated.
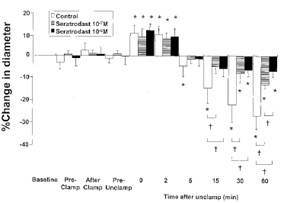
Figure 2. Effects of topical infusion of seratrodast on
reactivity of small cerebral (Cerebral microcirculation,
brain microcirculation)pial arterioles ( 75 m) to aortic
clamping and
unclamping in 21 rabbits. Data are expressed as percentage
change from the diameter measured just before topical
administration of
drug (baseline). Data are shown for 15 min after topical
administration (Pre-Clamp), just after clamping (After Clamp),
20 min after
clamping (Pre-Unclamp), and 0, 2, 5, 15, 30, and 60 min after
unclamping. Values are mean sd. *P 0.05 compared with baseline
in the same group; †P 0.05 as indicated.
Seratrodast has been reported to competitively inhibit
contractions of guinea pig tracheal strips and saphenous vein
strips in response to the TxA2 mimic, U-46619, but it has not
been reported to inhibit the contractions of tracheal strips
induced by leukotriene D4, platelet-activating factor, or
histamine (16). Seratrodast also competitively inhibits the
binding of [3H]U-46619 to Chinese hamster ovary cells into which
the TxA2 receptor-coding gene has been introduced and which
stably express the human TxA2 receptor (17). These findings
suggest that the pharmacologic effects of seratrodast are caused
by antagonism of TxA2 receptors, although additional use of
another chemically dissimilar TxA2 receptor antagonist may
potentiate the present finding. In the present study, we
administered seratrodast topically, not systemically, because we
desired to observe its direct effect on the cerebral
(Cerebral microcirculation,
brain microcirculation)circulation. It has been reported that pulmonary hypertension is
caused by TxA2 in humans after ischemia of the lower torso
during abdominal aortic aneurysmectomy (10,12). Thus, if we had
administered seratrodast systemically, the cerebral circulation
would likely have been affected by secondary effects of the drug
on the systemic circulation. In our experiment, seratrodast had
no detectable systemic effects when delivered beneath the
cranial window. Using the closed cranial window technique,
Haberl et al. (18) found that topical application of 10 6 M
U-46619 induced pial arteriolar vasoconstriction in rabbits
(maximal vasoconstriction of 9.7%). The present results, showing
a control decrease of 17%¨C28% in the diameter of pial arterioles
after aortic unclamping and a suppression by approximately half
with a TxA2 receptor antagonist, are consistent with the above
finding.
The initial vasodilation we observed in cerebral(Cerebral microcirculation,
brain microcirculation) pial arterioles
after unclamping could be caused by many factors, including the
accumulated CO2 in the blood returning from the ischemic area,
its low pH, and the dynamic cerebral(Cerebral microcirculation,
brain microcirculation) blood flow response to a
sudden decrease of PrAP. Liu et al. (1) noted that VMCA
increased significantly after unclamping of the infrarenal
abdominal aorta in anesthetized humans, and it paralleled the
change in Petco2. Likewise, other studies have indicated that
the increase in VMCA observed in humans for 10 minutes after
tourniquet deflation paralleled the change in Petco2 (19,20). In
the present study, Paco2 increased by approximately 5 mm Hg just
after unclamping (compared with the value obtained just before
unclamping) in all groups. Because we increased minute
ventilation to maintain Petco2 between 35 to 40 mm Hg just after
unclamping, any vasodilator stimulus to cerebral(Cerebral microcirculation,
brain microcirculation) pial vessels
resulting from the increase in Paco2 would probably have been
present for only a few minutes. Hyperventilation after
tourniquet deflation in humans seems to effectively prevent any
increase in VMCA (19), or indeed any prolonged changes in
arterial blood gas tension and pH. This suggests that our
adjustment of mechanical ventilation soon after unclamping would
have minimized the CO2-induced response of the pial vessels.
Although cerebral(Cerebral microcirculation,
brain microcirculation) neurologic complications related to abdominal
aortic surgery are not frequent, such complications could be
serious. The cerebral vasoconstriction observed in the present
study could imply that cerebral(Cerebral microcirculation,
brain microcirculation) ischemia may occur because of
microcirculatory failure after unclamping, and thus be critical
in the clinical setting. In patients who have a damaged
endothelium (such as those with atherosclerosis or
hypercholesterolemia), the presumed TxA2-induced responses of
cerebral(Cerebral microcirculation,
brain microcirculation) vessels to aortic unclamping could be different, and
possibly more pronounced. In fact, it has been reported that
endothelial damage induces arterial thrombosis via an increase
in TxA2 (21,22). Furthermore, a powerful cerebral(Cerebral microcirculation,
brain microcirculation)
vasoconstriction may be induced after rupture of an abdominal
aortic aneurysm, because vasospastic mediators may be produced
in large amounts in hemorrhagic shock (14).
Because we did not monitor the cerebral (Cerebral microcirculation,
brain microcirculation)blood flow, we cannot
comment about changes after aortic unclamping. However, we
measured the diameters of pial arterioles that reflect
conductance of important segments of the cerebral (Cerebral microcirculation,
brain microcirculation)microvascular
bed. A method for implantation of the cranial window makes it
possible to observe the microcirculation(Cerebral microcirculation,
brain microcirculation) directly and to measure
the diameter of pial vessels accurately. This method also
permits study of the effects on the microcirculation(Cerebral microcirculation,
brain microcirculation) of a
variety of maneuvers and vasoactive drugs that can be evaluated
by direct application as well as by intravascular
administration. If upstream and downstream pressures did not
change, a proportionate change in flow would occur. Because it
preserves the integrity of the skull, this technique allows
study of the cerebral microcirculation
(brain microcirculation)under conditions closely
approximating the normal environment of cerebral(Cerebral microcirculation,
brain microcirculation) vessels (23).
Conversely, the cerebral (Cerebral microcirculation,
brain microcirculation)circulation is heterogeneous and this
method may not predict overall cerebral (Cerebral microcirculation,
brain microcirculation)blood flow. Thus, we
cannot completely
exclude the possibility that the observed effects of pial vessel
reactivity induced by aortic clamping and unclamping might be
limited to the pial level,although pial arteriolar diameter
measurement is one of the ideal methods for studying
microvascular reactivity. In conclusion, pial arteriolar
vasoconstriction was induced by release of a 20-minute aortic
cross-clamp in anesthetized rabbits. This vasoconstriction is
partly induced via a washout of the TxA2 produced in the
ischemic region during clamping and after crossclamp release.
References
1. Liu G, Burcev I, Pott F, et al. Middle cerebral (Cerebral microcirculation,
brain microcirculation)artery flow
velocity and cerebral oxygenation during abdominal aortic
surgery. Anaesth Intensive Care 1999;27:148¨C53.
2. Bowald S, Gerdin B. Pulmonary microembolism during and after
aortic cross-clamping in heparinized and non-heparinized pigs.
Acta Chir Scand 1980;146:351¨C6.
3. Huval WV, Lelcuk S, Allen PD, et al. Determinants of
cardiovascular
stability during abdominal aortic aneurysmectomy (AAA). Ann Surg
1984;199:216¨C22. |